Question: Problem 3 - Equal A Priori Probabilities for a Perfect Gas. The molecules of air in our virtual classroom are, to a very good approximation,
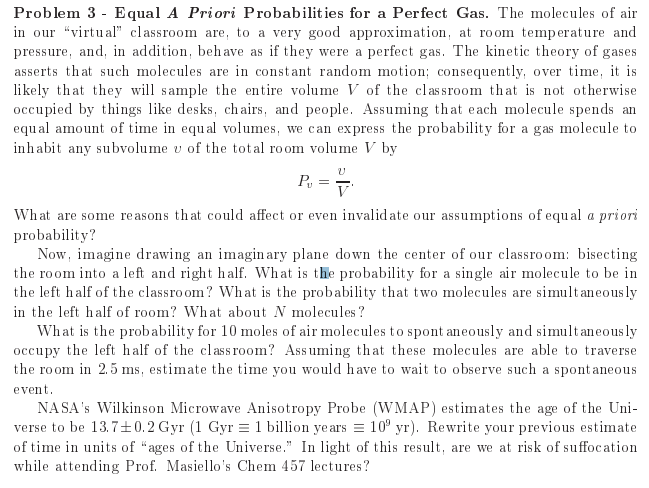
Problem 3 - Equal A Priori Probabilities for a Perfect Gas. The molecules of air in our "virtual" classroom are, to a very good approximation, at room temperature and pressure, and, in addition, behave as if they were a perfect gas. The kinetic theory of gases asserts that such molecules are in constant random motion; consequently, over time, it is likely that they will sample the entire volume V of the classroom that is not otherwise occupied by things like desks, chairs, and people. Assuming that each molecule spends an equal amount of time in equal volumes, we can express the probability for a gas molecule to inhabit any subvolume v of the total room volume V by P, What are some reasons that could affect or even invalidate our assumptions of equal a priori probability? Now, imagine drawing an imaginary plane down the center of our classroom: bisecting the room into a left and right half. What is the probability for a single air molecule to be in the left half of the classroom? What is the probability that two molecules are simultaneously in the left half of room? What about N molecules? What is the probability for 10 moles of air molecules to spontaneously and simultaneously occupy the left half of the classroom? Assuming that these molecules are able to traverse the room in 2.5 ms, estimate the time you would have to wait to observe such a spontaneous event. NASA's Wilkinson Microwave Anisotropy Probe (WMAP) estimates the age of the Uni- verse to be 13.7+0.2 Gyr (1 Gyr = 1 billion years = 10 yr). Rewrite your previous estimate of time in units of "ages of the Universe." In light of this result, are we at risk of suffocation while attending Prof. Masiello's Chem 457 lectures? Problem 3 - Equal A Priori Probabilities for a Perfect Gas. The molecules of air in our "virtual" classroom are, to a very good approximation, at room temperature and pressure, and, in addition, behave as if they were a perfect gas. The kinetic theory of gases asserts that such molecules are in constant random motion; consequently, over time, it is likely that they will sample the entire volume V of the classroom that is not otherwise occupied by things like desks, chairs, and people. Assuming that each molecule spends an equal amount of time in equal volumes, we can express the probability for a gas molecule to inhabit any subvolume v of the total room volume V by P, What are some reasons that could affect or even invalidate our assumptions of equal a priori probability? Now, imagine drawing an imaginary plane down the center of our classroom: bisecting the room into a left and right half. What is the probability for a single air molecule to be in the left half of the classroom? What is the probability that two molecules are simultaneously in the left half of room? What about N molecules? What is the probability for 10 moles of air molecules to spontaneously and simultaneously occupy the left half of the classroom? Assuming that these molecules are able to traverse the room in 2.5 ms, estimate the time you would have to wait to observe such a spontaneous event. NASA's Wilkinson Microwave Anisotropy Probe (WMAP) estimates the age of the Uni- verse to be 13.7+0.2 Gyr (1 Gyr = 1 billion years = 10 yr). Rewrite your previous estimate of time in units of "ages of the Universe." In light of this result, are we at risk of suffocation while attending Prof. Masiello's Chem 457 lectures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


