Question: Problem 3 . In a small - scale mining project in Kalinga, mining wastewater containing lead ions were removed using zeolite granules before the wastewater
Problem In a smallscale mining project in Kalinga, mining wastewater containing lead ions
were removed using zeolite granules before the wastewater can be safely disposed of in a river
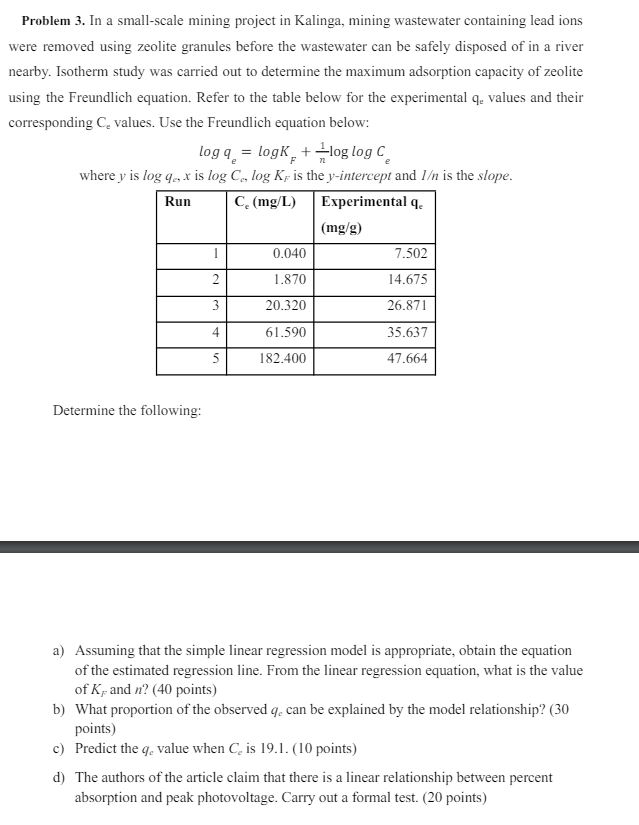
nearby. Isotherm study was carried out to determine the maximum adsorption capacity of zeolite
using the Freundlich equation. Refer to the table below for the experimental values and their
corresponding values. Use the Freundlich equation below:
where is is is the intercept and is the slope.
Determine the following:
a Assuming that the simple linear regression model is appropriate, obtain the equation
of the estimated regression line. From the linear regression equation, what is the value
of and points
b What proportion of the observed can be explained by the model relationship?
points
c Predict the value when is points
d The authors of the article claim that there is a linear relationship between percent
absorption and peak photovoltage. Carry out a formal test. point
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


