Question: Problem 3.37. Consider a monatomic ideal gas that lives at a height z above sea level, so each molecule has potential energy myz in addition
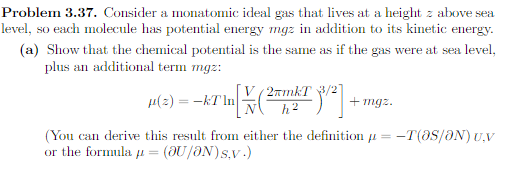
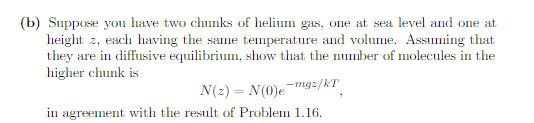


Problem 3.37. Consider a monatomic ideal gas that lives at a height z above sea level, so each molecule has potential energy myz in addition to its kinetic energy. (a) Show that the chemical potential is the same as if the gas were at sea level, plus an additional term mgz: V 2mmkT 3/2 "(=) = -kT In N h2 + mgz. (You can derive this result from either the definition a = -T(OS/ON) U.V or the formula p = (OU/ON)s.v. )(b) Suppose you have two chunks of helium gas, one at sea level and one at height z, each having the same temperature and volume. Assuming that they are in diffusive equilibrium, show that the number of molecules in the higher chunk is N(2) = NO)e -mg:/KT in agreement with the result of Problem 1.16.Problem 1.16. The exponential atmosphere. (a) Consider a horizontal slab of air whose thickness (height) is de. If this slab is at rest. the pressure holding it up from below must balance both the pressure from above and the weight of the slab. Use this fact to find an expression for dP/de, the variation of pressure with altitude, in terms of the density of air. (b) Use the ideal gas law to write the density of air in terms of pressure, tem- perature, and the average mass m of the air molecules. (The information needed to calculate m is given in Problem 1.14.) Show, then, that the pressure obeys the differential equation dP dz P. KT called the barometric equation. (c) Assuming that the temperature of the atmosphere is independent of height ( not a great assumption but not terrible either), solve the barometric equa- tion to obtain the pressure as a function of height: P(z) = P(0)e-mg=/KT Show also that the density obeys a similar equation.(d) Estimate the pressure, in atmospheres, at the following locations: Ogden, Utah (4700 ft or 1430 m above sea level); Leadville, Colorado (10,150 ft, 3090 m); Mt. Whitney, California (14,500 ft, 4420 m); Mt. Everest, Nepal/ Tibet (29,000 ft, 8850 m). (Assume that the pressure at sea level is 1 atm.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


