Question: Problem 4 -10 marks Ammonia is burned to form nitric oxide in the following reaction: 4NH3+5O24NO+6H2O (a) Calculate the ratio (lb-mole O2 react/lb-mole NO formed).
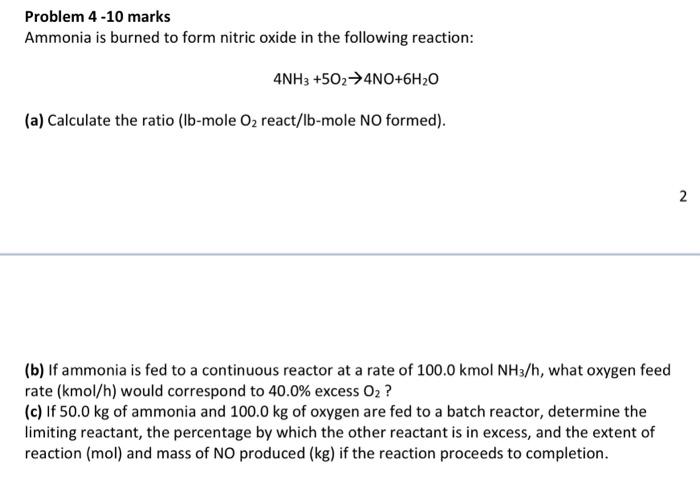
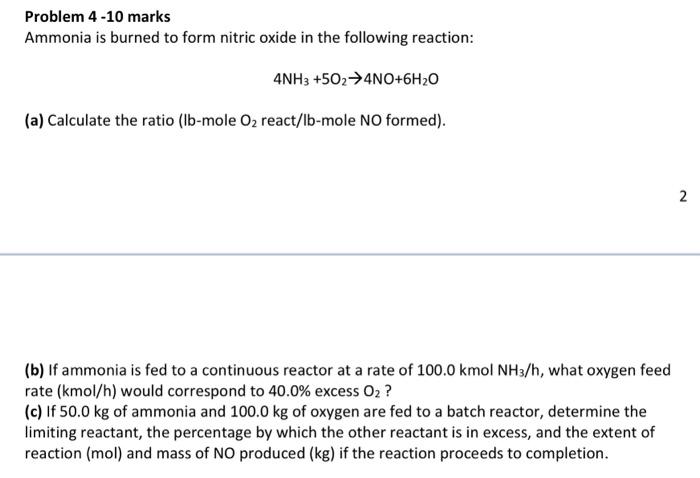
Problem 4 -10 marks Ammonia is burned to form nitric oxide in the following reaction: 4NH3+5O24NO+6H2O (a) Calculate the ratio (lb-mole O2 react/lb-mole NO formed). (b) If ammonia is fed to a continuous reactor at a rate of 100.0kmolNH3/h, what oxygen feed rate (kmol/h) would correspond to 40.0% excess O2 ? (c) If 50.0kg of ammonia and 100.0kg of oxygen are fed to a batch reactor, determine the limiting reactant, the percentage by which the other reactant is in excess, and the extent of reaction (mol) and mass of NO produced (kg) if the reaction proceeds to completion. Problem 4 -10 marks Ammonia is burned to form nitric oxide in the following reaction: 4NH3+5O24NO+6H2O (a) Calculate the ratio (lb-mole O2 react/lb-mole NO formed). (b) If ammonia is fed to a continuous reactor at a rate of 100.0kmolNH3/h, what oxygen feed rate (kmol/h) would correspond to 40.0% excess O2 ? (c) If 50.0kg of ammonia and 100.0kg of oxygen are fed to a batch reactor, determine the limiting reactant, the percentage by which the other reactant is in excess, and the extent of reaction (mol) and mass of NO produced (kg) if the reaction proceeds to completion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


