Question: Problem 4. A receipt of surface water sample is given in Table below: It is known that phenolphthalein alkalinity is only due to CO32 and
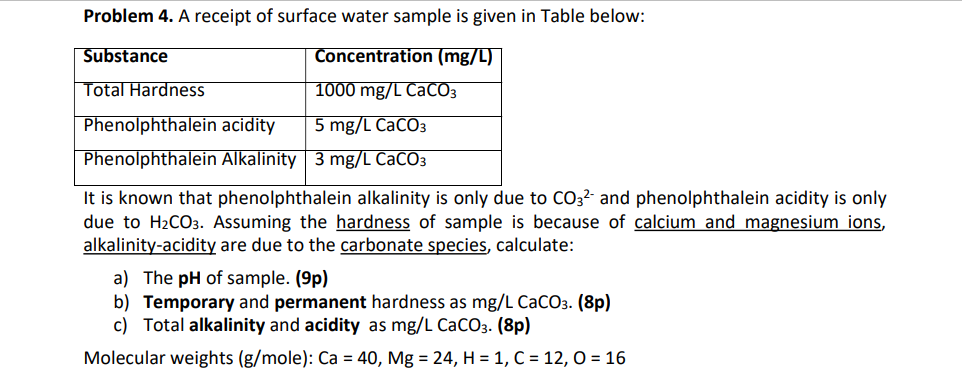
Problem 4. A receipt of surface water sample is given in Table below: It is known that phenolphthalein alkalinity is only due to CO32 and phenolphthalein acidity is only due to H2CO3. Assuming the hardness of sample is because of calcium and magnesium ions, alkalinity-acidity are due to the carbonate species, calculate: a) The pH of sample. (9p) b) Temporary and permanent hardness as mg/LCaCO3. (8p) c) Total alkalinity and acidity asmg/LCaCO3. (8p) Molecular weights (g/mole): Ca=40,Mg=24,H=1,C=12,O=16
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


