Question: Problem 4. According to the Kelvin-Plank statement, we cannot make a device that will operate in a cycle and produce no effects other than doing
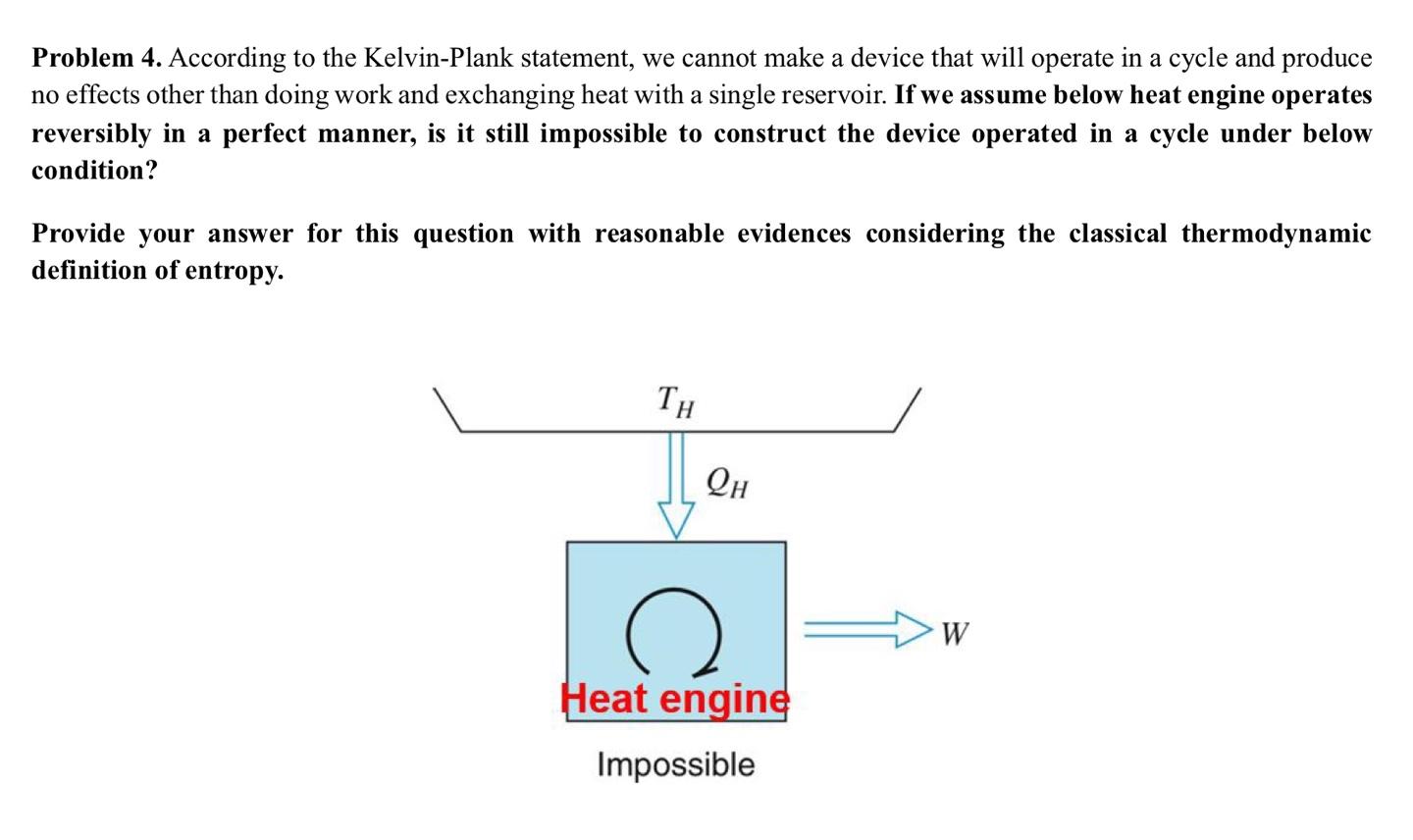
Problem 4. According to the Kelvin-Plank statement, we cannot make a device that will operate in a cycle and produce no effects other than doing work and exchanging heat with a single reservoir. If we assume below heat engine operates reversibly in a perfect manner, is it still impossible to construct the device operated in a cycle under below condition? Provide your answer for this question with reasonable evidences considering the classical thermodynamic definition of entropy. TH QH W Heat engine Impossible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


