Question: Problem 4: (Interfacial effects) (20 points) 1. A spherical precipitate of B forms in an a matrix in a Pb-Sn alloy. The molar volume of
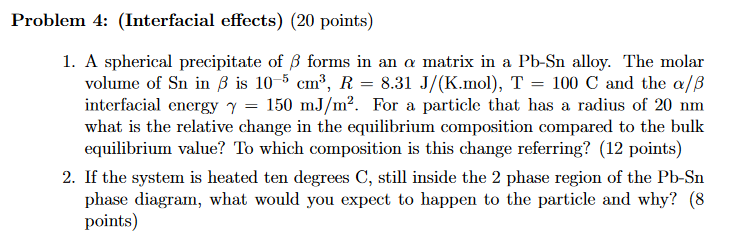
Problem 4: (Interfacial effects) (20 points) 1. A spherical precipitate of B forms in an a matrix in a Pb-Sn alloy. The molar volume of Sn in B is 10-5 cm, R = 8.31 J/(K.mol), T = 100 C and the a/B interfacial energy 7 = 150 mJ/m. For a particle that has a radius of 20 nm what is the relative change in the equilibrium composition compared to the bulk equilibrium value? To which composition is this change referring? (12 points) 2. If the system is heated ten degrees C, still inside the 2 phase region of the Pb-Sn phase diagram, what would you expect to happen to the particle and why? (8 points) Problem 4: (Interfacial effects) (20 points) 1. A spherical precipitate of B forms in an a matrix in a Pb-Sn alloy. The molar volume of Sn in B is 10-5 cm, R = 8.31 J/(K.mol), T = 100 C and the a/B interfacial energy 7 = 150 mJ/m. For a particle that has a radius of 20 nm what is the relative change in the equilibrium composition compared to the bulk equilibrium value? To which composition is this change referring? (12 points) 2. If the system is heated ten degrees C, still inside the 2 phase region of the Pb-Sn phase diagram, what would you expect to happen to the particle and why? (8 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


