Question: Problem #4: The excess Gibbs free energy for the binary system chloroform (1)/ethanol (2) at 55C is well represented by the Margules equation: GEx/RT=(1.42x1+0.59x2)x1x2 The
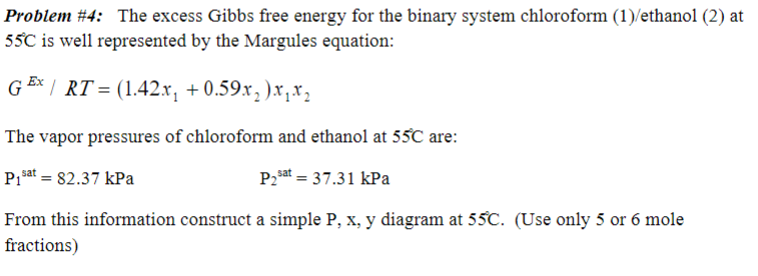
Problem \#4: The excess Gibbs free energy for the binary system chloroform (1)/ethanol (2) at 55C is well represented by the Margules equation: GEx/RT=(1.42x1+0.59x2)x1x2 The vapor pressures of chloroform and ethanol at 55C are: P1sat=82.37kPaP2sat=37.31kPa From this information construct a simple P,x,y diagram at 55C. (Use only 5 or 6 mole fractions)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


