Question: >Problem 4.2-3 Valve State 1 Nozzle Inlet State 2 Nozzle Exit 689 kPa and 17. P2 = 389 C t2 = ??? Superheated Steam 689
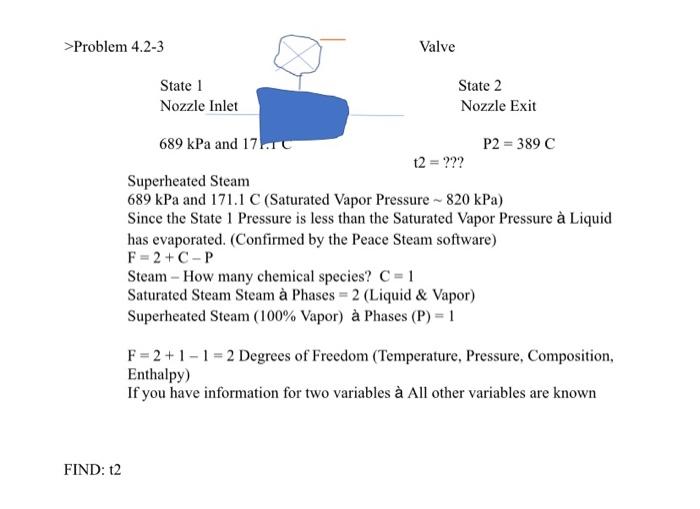
>Problem 4.2-3 Valve State 1 Nozzle Inlet State 2 Nozzle Exit 689 kPa and 17. P2 = 389 C t2 = ??? Superheated Steam 689 kPa and 171.1 C (Saturated Vapor Pressure - 820 kPa) Since the State 1 Pressure is less than the Saturated Vapor Pressure Liquid has evaporated. (Confirmed by the Peace Steam software) F = 2 + C-P Steam - How many chemical species? C = 1 Saturated Steam Steam Phases = 2 (Liquid & Vapor) Superheated Steam (100% Vapor) Phases (P) = 1 F = 2 + 1 - 1 = 2 Degrees of Freedom (Temperature, Pressure, Composition, Enthalpy) If you have information for two variables All other variables are known FIND: 12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


