Question: Problem 5 Gaseous propane ( left ( mathrm { C } _ { 3 } mathrm { H } _ {
Problem
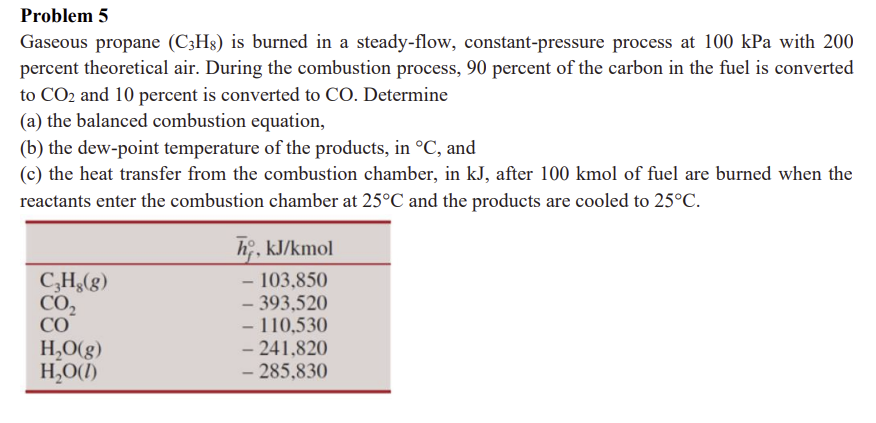
Gaseous propane leftmathrmCmathrmHright is burned in a steadyflow, constantpressure process at kPa with percent theoretical air. During the combustion process, percent of the carbon in the fuel is converted to mathrmCO and percent is converted to CO Determine
a the balanced combustion equation,
b the dewpoint temperature of the products, in circmathrmC and
c the heat transfer from the combustion chamber, in kJ after kmol of fuel are burned when the reactants enter the combustion chamber at circmathrmC and the products are cooled to circmathrmC
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


