Question: Problem 5-6 (Level 1) The overall reaction for the formation of diethyl ether from ethanol is 2C2H5OH = (C2H5)20 + H2O (2A = E +
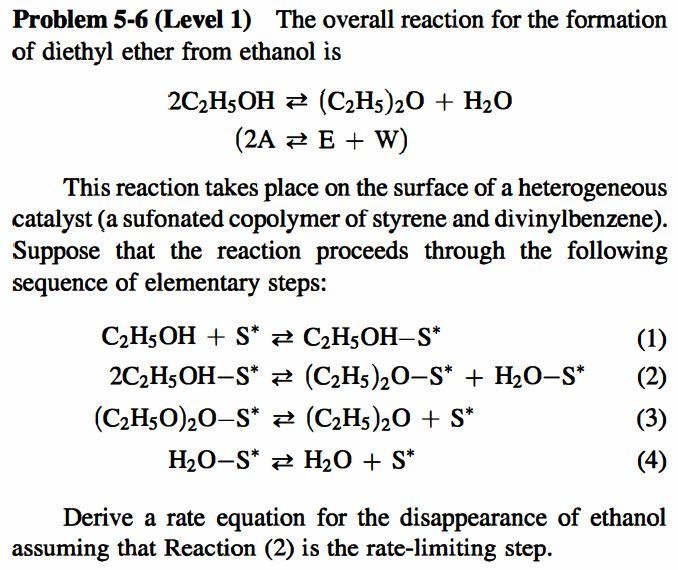
Problem 5-6 (Level 1) The overall reaction for the formation of diethyl ether from ethanol is 2C2H5OH = (C2H5)20 + H2O (2A = E + W) This reaction takes place on the surface of a heterogeneous catalyst (a sufonated copolymer of styrene and divinylbenzene). Suppose that the reaction proceeds through the following sequence of elementary steps: (1) C2H5OH + S* # C2H5OH-S* 2C2H5OH-S* = (C2H5)20-5* + H20-S* (C2H50)20-S* # (C2H5)20 + S* H2O-s* H2O + S* (3) (4) Derive a rate equation for the disappearance of ethanol assuming that Reaction (2) is the rate-limiting step
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


