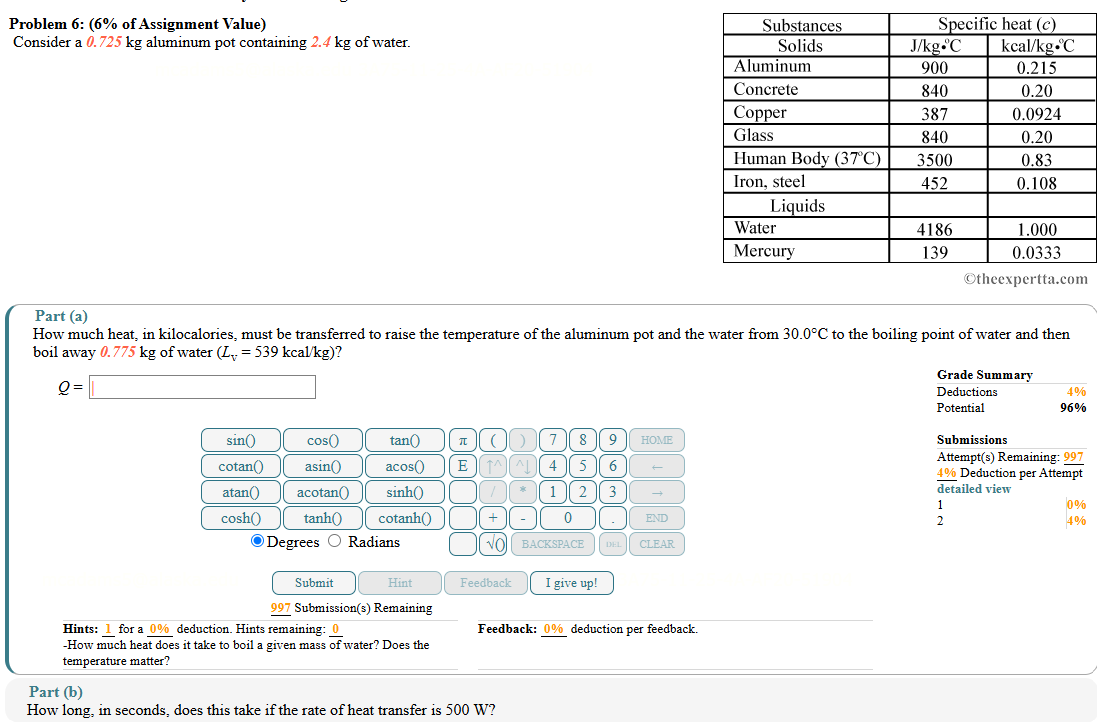
Question: Problem 6 : ( 6 % of Assignment Value ) Consider a 0 . 7 2 5 kg aluminum pot containing 2 . 4
Problem : of Assignment Value
Consider a kg aluminum pot containing kg of water.
Otheexperttacom
Part a
How much heat, in kilocalories, must be transferred to raise the temperature of the aluminum pot and the water from circmathrmC to the boiling point of water and then boil away kg of water Lmathrmvmathrmkcalmathrmkg
Qsquare
Grade Summary
Deductions
Potential
Submissions
Attempts Remaining: Deduction per Attempt detailed view
Degrees
Radians
Submissions Remaining
Hints: for a deduction. Hints remaining:
How much heat does it take to boil a given mass of water? Does the
Feedback: deduction per feedback. temperature matter?
Part b
How long, in seconds, does this take if the rate of heat transfer is W
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


