Question: Problem 7-10 (Level 1) An ideal batch reactor is to be sized for the polymerization of styrene monomer to polystyrene. Pure styrene monomer containing a
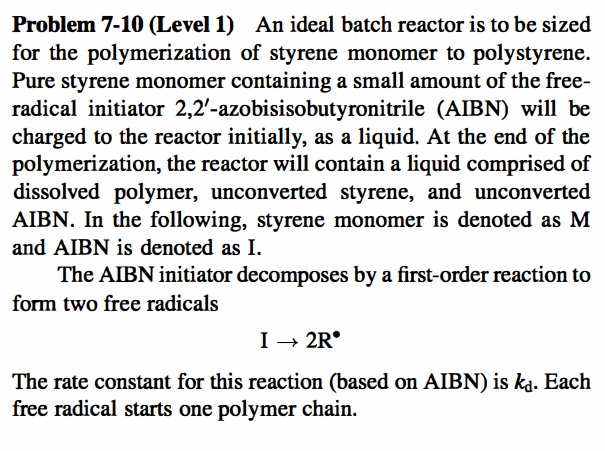
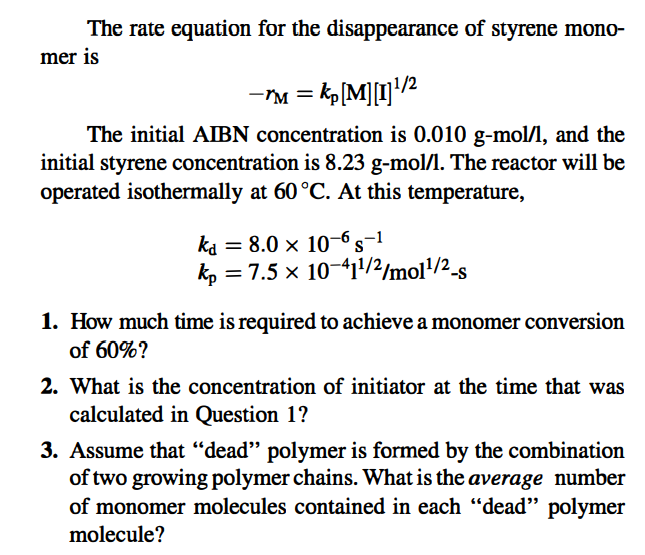
Problem 7-10 (Level 1) An ideal batch reactor is to be sized for the polymerization of styrene monomer to polystyrene. Pure styrene monomer containing a small amount of the free- radical initiator 2,2'-azobisisobutyronitrile (AIBN) will be charged to the reactor initially, as a liquid. At the end of the polymerization, the reactor will contain a liquid comprised of dissolved polymer, unconverted styrene, and unconverted AIBN. In the following, styrene monomer is denoted as M and AIBN is denoted as I. The AIBN initiator decomposes by a first-order reaction to form two free radicals I + 2R The rate constant for this reaction (based on AIBN) is kd. Each free radical starts one polymer chain. The rate equation for the disappearance of styrene mono- mer is -*M = kp[M][I]"/2 The initial ABN concentration is 0.010 g-mol/l, and the initial styrene concentration is 8.23 g-mol/l. The reactor will be operated isothermally at 60 C. At this temperature, ka = 8.0 x 10-6s-1 kp = 7.5 x 10-411/2/mo11/2_s -S 1. How much time is required to achieve a monomer conversion of 60%? 2. What is the concentration of initiator at the time that was calculated in Question 1? 3. Assume that "dead" polymer is formed by the combination of two growing polymer chains. What is the average number of monomer molecules contained in each dead polymer molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


