Question: Problem 7.10 The concentration of a reactant in a first-order chemical reaction that proceeds at a rate & can be described as follows: In C
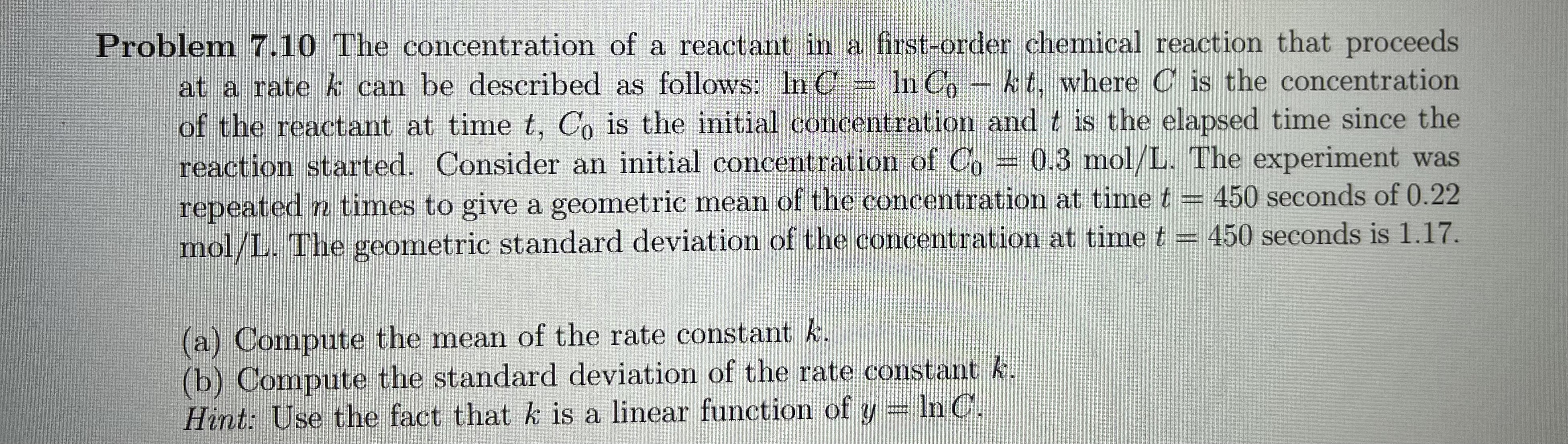
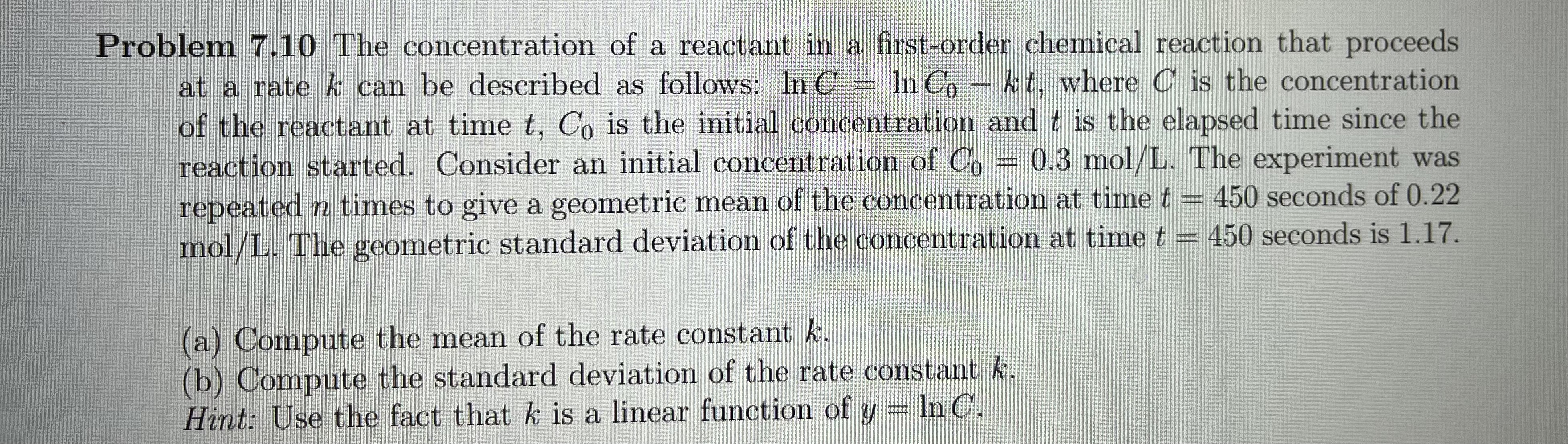
Problem 7.10 The concentration of a reactant in a first-order chemical reaction that proceeds at a rate & can be described as follows: In C = In Co - kt, where C is the concentration of the reactant at time t, Co is the initial concentration and t is the elapsed time since the reaction started. Consider an initial concentration of Co = 0.3 mol/L. The experiment was repeated n times to give a geometric mean of the concentration at time t = 450 seconds of 0.22 mol/L. The geometric standard deviation of the concentration at time t = 450 seconds is 1.17. (a) Compute the mean of the rate constant k. (b) Compute the standard deviation of the rate constant k. Hint: Use the fact that k is a linear function of y = In C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


