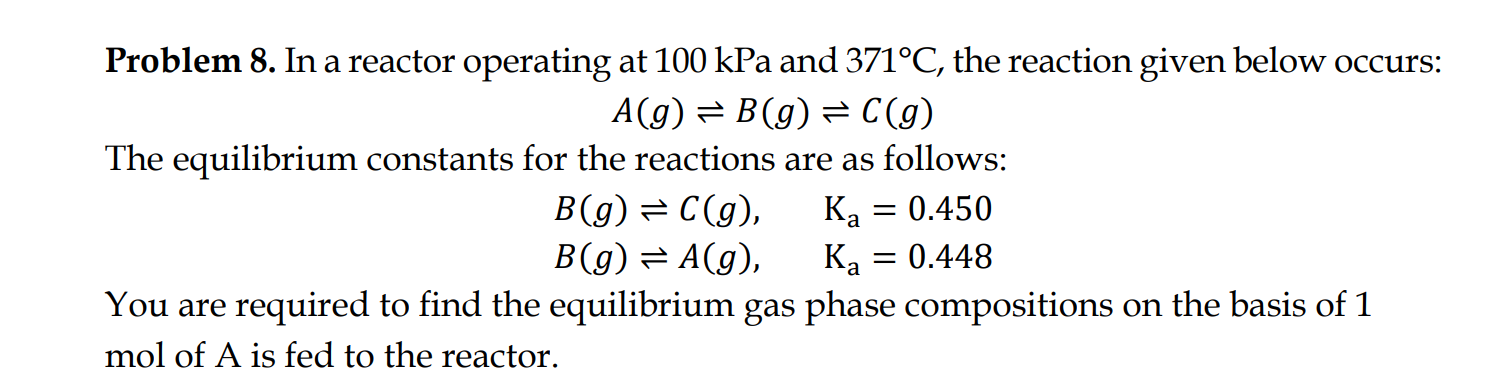
Question: Problem 8 . In a reactor operating at 1 0 0 kPa and 3 7 1 C , the reaction given below occurs: A (
Problem In a reactor operating at kPa and the reaction given below occurs:
The equilibrium constants for the reactions are as follows:
You are required to find the equilibrium gas phase compositions on the basis of
mol of is fed to the reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


