Question: Problem: A gas undergoes 3 process that completes a cycle in series P1= 10 atm V1=4 m3 PV1.1-constant Q=0 P2= 50 atm V2= ? P=K
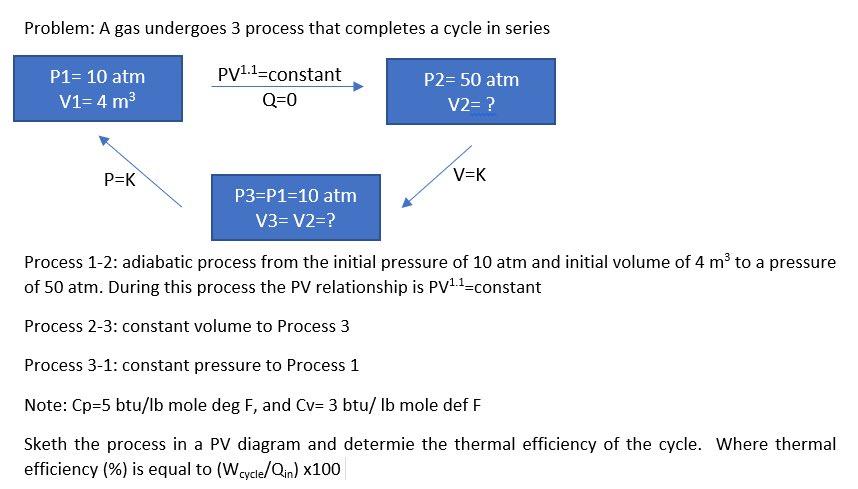
Problem: A gas undergoes 3 process that completes a cycle in series P1= 10 atm V1=4 m3 PV1.1-constant Q=0 P2= 50 atm V2= ? P=K V=K P3=P1=10 atm V3= V2=? Process 1-2: adiabatic process from the initial pressure of 10 atm and initial volume of 4 m to a pressure of 50 atm. During this process the PV relationship is PV1.1-constant Process 2-3: constant volume to Process 3 Process 3-1: constant pressure to Process 1 Note: Cp=5 btu/lb mole deg F, and Cv= 3 btu/ lb mole def F Sketh the process in a PV diagram and determie the thermal efficiency of the cycle. Where thermal efficiency (%) is equal to (W cycle/Qin) x100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


