Question: Problem: Consider the following catalytic data reported in a recent publication. What information is missing? Table 1 Biphasic hydrogenation reactions of arenes in the [bmim][BF4]
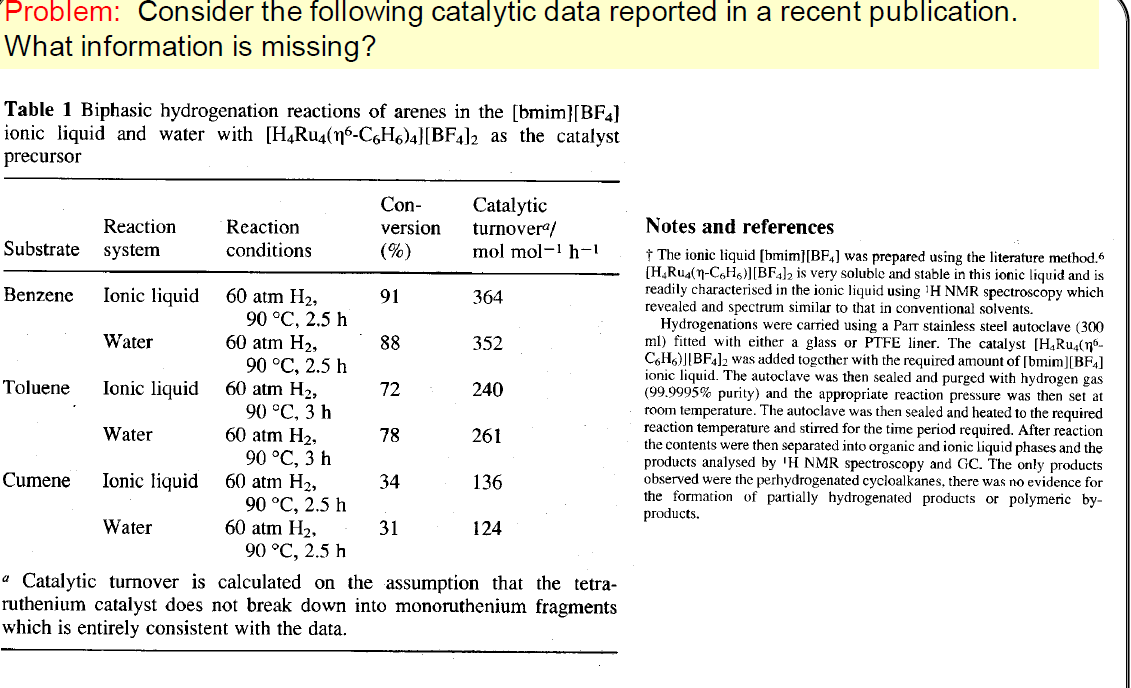
Problem: Consider the following catalytic data reported in a recent publication. What information is missing? Table 1 Biphasic hydrogenation reactions of arenes in the [bmim][BF4] ionic liquid and water with [H_Ru4(76-C6H6)4][BF4]2 as the catalyst precursor Reaction system Reaction conditions Con- version (%) Catalytic turnovera/ mol mol-1 h-1 Substrate Benzene Ionic liquid 60 atm H2, 91 364 90 C, 2.5 h Water 60 atm H2, 88 352 90 C, 2.5 h Toluene Ionic liquid 60 atm H2, 72 240 90 C, 3 h Water 60 atm H2, 78 261 90 C, 3 h Cumene Ionic liquid 60 atm H2, 34 136 90 C, 2.5 h Water 60 atm H2, 31 124 90 C, 2.5 h a Catalytic turnover is calculated on the assumption that the tetra- ruthenium catalyst does not break down into monoruthenium fragments which is entirely consistent with the data. Notes and references The ionic liquid [hmim][BF4] was prepared using the literature method. 6 [H.Ru (n-C6H)][BF4]2 is very soluble and stable in this ionic liquid and is readily characterised in the ionic liquid using 'H NMR spectroscopy which revealed and spectrum similar to that in conventional solvents. Hydrogenations were carried using a Part stainless steel autoclave (300 ml) fitted with either a glass or PTFE liner. The catalyst [H.Ru.(no_ C.H.)][BF4]2 was added together with the required amount of [bmim][BF4] ionic liquid. The autoclave was then sealed and purged with hydrogen gas (99.9995% purity) and the appropriate reaction pressure was then set at room temperature. The autoclave was then sealed and heated to the required reaction temperature and stirred for the time period required. After reaction the contents were then separated into organic and ionic liquid phases and the products analysed by 'H NMR spectroscopy and GC. The only products observed were the perhydrogenated cycloalkanes, there was no evidence for the formation of partially hydrogenated products or polymeric by- products. Problem: Consider the following catalytic data reported in a recent publication. What information is missing? Table 1 Biphasic hydrogenation reactions of arenes in the [bmim][BF4] ionic liquid and water with [H_Ru4(76-C6H6)4][BF4]2 as the catalyst precursor Reaction system Reaction conditions Con- version (%) Catalytic turnovera/ mol mol-1 h-1 Substrate Benzene Ionic liquid 60 atm H2, 91 364 90 C, 2.5 h Water 60 atm H2, 88 352 90 C, 2.5 h Toluene Ionic liquid 60 atm H2, 72 240 90 C, 3 h Water 60 atm H2, 78 261 90 C, 3 h Cumene Ionic liquid 60 atm H2, 34 136 90 C, 2.5 h Water 60 atm H2, 31 124 90 C, 2.5 h a Catalytic turnover is calculated on the assumption that the tetra- ruthenium catalyst does not break down into monoruthenium fragments which is entirely consistent with the data. Notes and references The ionic liquid [hmim][BF4] was prepared using the literature method. 6 [H.Ru (n-C6H)][BF4]2 is very soluble and stable in this ionic liquid and is readily characterised in the ionic liquid using 'H NMR spectroscopy which revealed and spectrum similar to that in conventional solvents. Hydrogenations were carried using a Part stainless steel autoclave (300 ml) fitted with either a glass or PTFE liner. The catalyst [H.Ru.(no_ C.H.)][BF4]2 was added together with the required amount of [bmim][BF4] ionic liquid. The autoclave was then sealed and purged with hydrogen gas (99.9995% purity) and the appropriate reaction pressure was then set at room temperature. The autoclave was then sealed and heated to the required reaction temperature and stirred for the time period required. After reaction the contents were then separated into organic and ionic liquid phases and the products analysed by 'H NMR spectroscopy and GC. The only products observed were the perhydrogenated cycloalkanes, there was no evidence for the formation of partially hydrogenated products or polymeric by- products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


