Question: Problem Raw groundwater with analysis as tabulated below is to be softeried by an excess lime - soda process. a ) Compute the concentration of
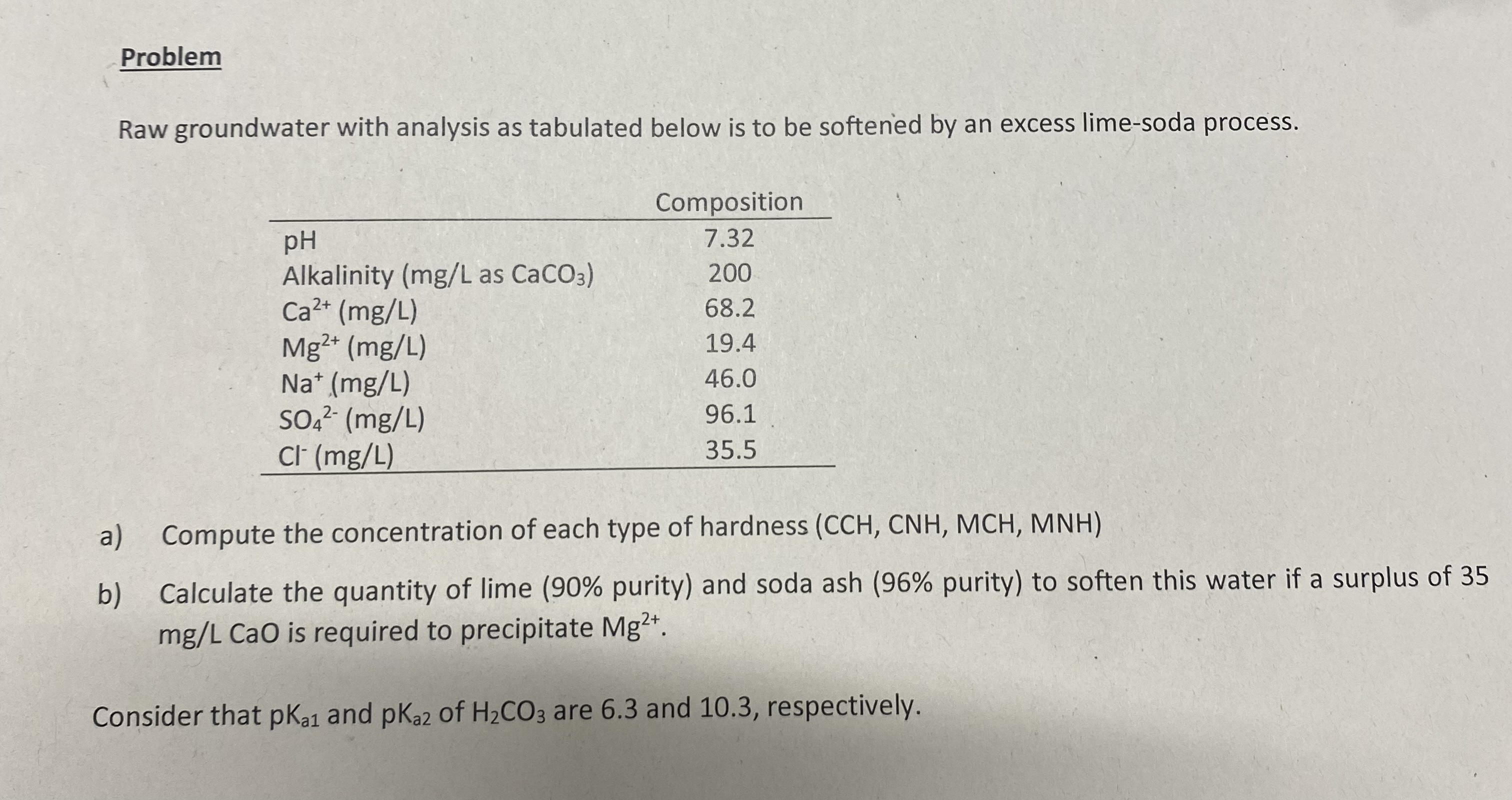
Problem
Raw groundwater with analysis as tabulated below is to be softeried by an excess limesoda process.
a Compute the concentration of each type of hardness
b Calculate the quantity of lime purity and soda ash purity to soften this water if a surplus of
CaO is required to precipitate
Consider that and of are and respectively.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


