Question: PROBLEM SET 2: GEOMETRY 10.2 Sketch the shape of a linear triatomic molecule, a trigo- 10.24 Arrange the following compounds in order of innal planar
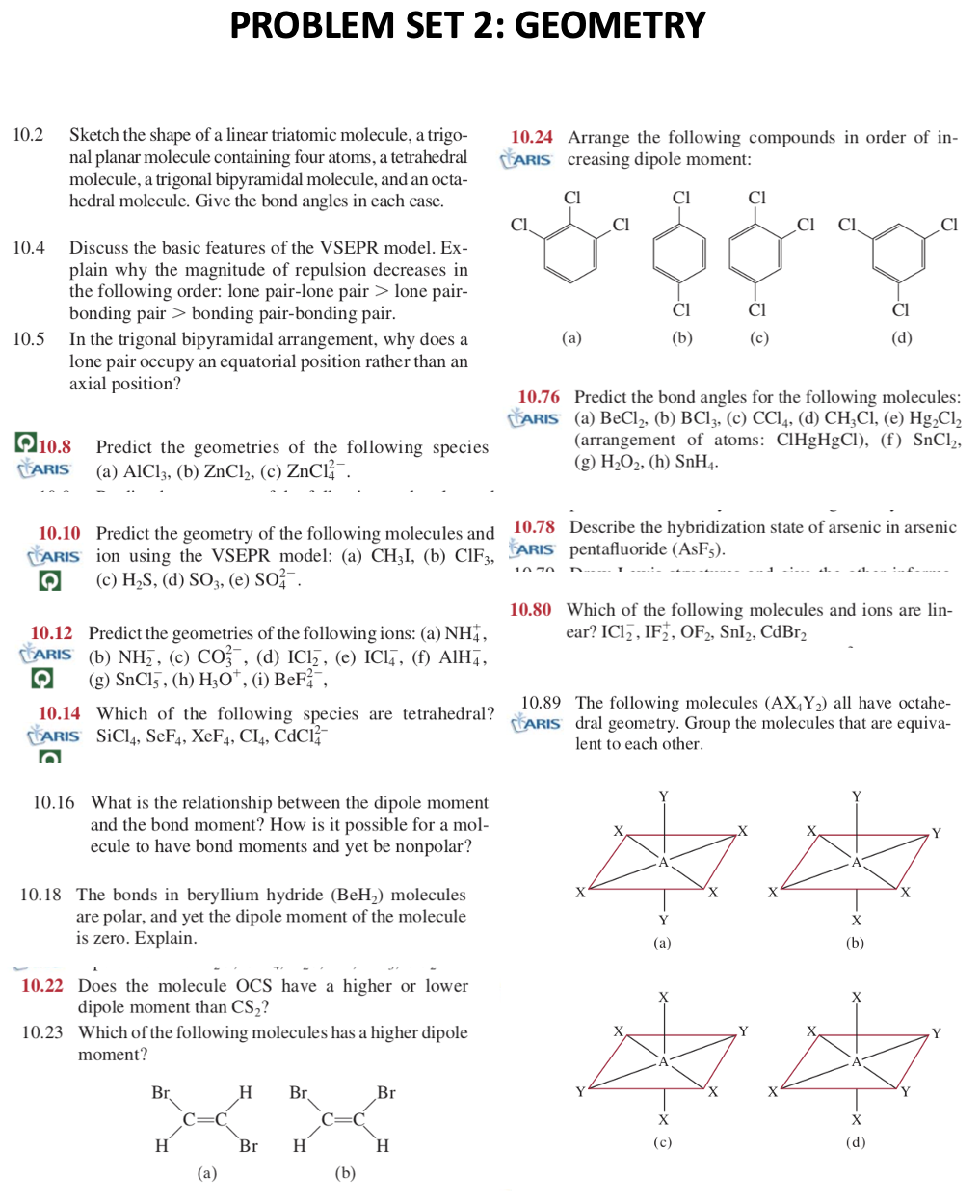
PROBLEM SET 2: GEOMETRY 10.2 Sketch the shape of a linear triatomic molecule, a trigo- 10.24 Arrange the following compounds in order of innal planar molecule containing four atoms, a tetrahedral CARIS creasing dipole moment: molecule, a trigonal bipyramidal molecule, and an octahedral molecule. Give the bond angles in each case. 10.4 Discuss the basic features of the VSEPR model. Explain why the magnitude of repulsion decreases in the following order: lone pair-lone pair > lone pairbonding pair > bonding pair-bonding pair. 10.5 In the trigonal bipyramidal arrangement, why does a (a) (b) (c) (d) lone pair occupy an equatorial position rather than an axial position? 10.76 Predict the bond angles for the following molecules: CaRIS (a) BeCl2, (b) BCl3, (c) CCl4, (d) CH3Cl, (e) Hg2Cl2 Q10.8 Predict the geometries of the following species (arrangement of atoms: ClHgHgCl2, (f) SnCl2, (aRIS AlCl3, (b) ZnCl2, (c) ZnCl42. (g) H2O2, (h) SnH4. 10.10 Predict the geometry of the following molecules and 10.78 Describe the hybridization state of arsenic in arsenic CARIS ion using the VSEPR model: (a) CH3I, (b) ClF3, IARIS pentafluoride (AsF5 ). (c) H2S, (d) SO3, (e) SO42. CARIS (b) NH2, (c) CO32, (d) ICl2, (e) ICl4, (f) AlH4, (g) SnCl5, (h) H3O+, (i) BeF42, ear? ICl2,IF2+,OF2,SnI2,CdBr2 10.14 Which of the following species are tetrahedral? 10.89 The following molecules (AX4Y2) all have octaheCARIS SiCl4,SeF4,XeF4,CI4,CdCl42 WARIS dral geometry. Group the molecules that are equiva lent to each other. 10.16 What is the relationship between the dipole moment and the bond moment? How is it possible for a molecule to have bond moments and yet be nonpolar? 10.18 The bonds in beryllium hydride (BeH2) molecules are polar, and yet the dipole moment of the molecule is zero. Explain. 10.22 Does the molecule OCS have a higher or lower dipole moment than CS2 ? 10.23 Which of the following molecules has a higher dipole moment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


