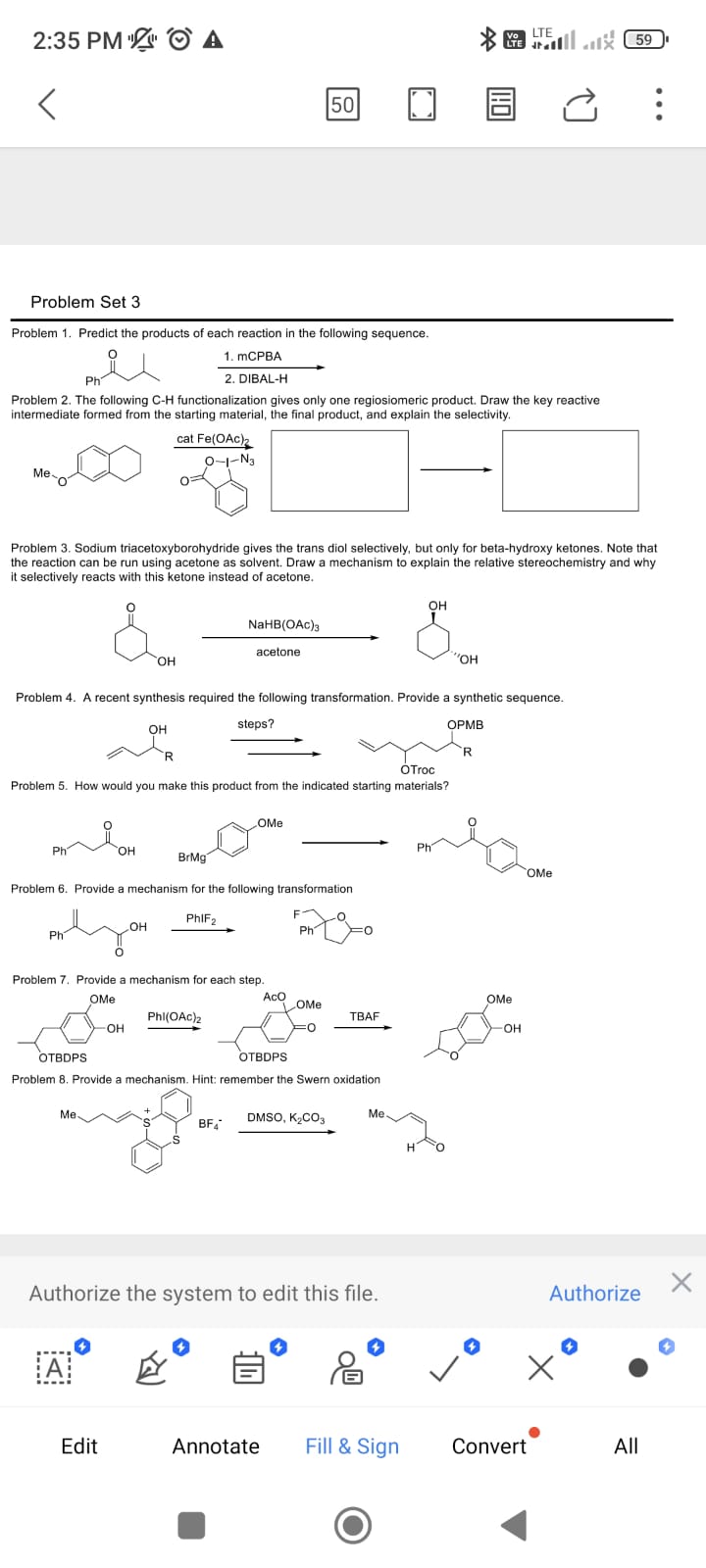
Question: Problem Set 3 Problem 1 . Predict the products of each reaction in the following sequence. ? 2 . D I B A L -
Problem Set
Problem Predict the products of each reaction in the following sequence.
Problem The following functionalization gives only one regiosiomeric product. Draw the key reactive
intermediate formed from the starting material, the final product, and explain the selectivity.
Problem Sodium triacetoxyborohydride gives the trans diol selectively, but only for betahydroxy ketones. Note that
the reaction can be run using acetone as solvent. Draw a mechanism to explain the relative stereochemistry and why
it selectively reacts with this ketone instead of acetone.
Problem A recent synthesis required the following transformation. Provide a synthetic sequence.
Problem Provide a mechanism for the following transformation
Problem Provide a mechanism. Hint: remember the Swern oxidation
Authorize the system to edit this file.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


