Question: Problem Situation 1: Carbon Dating When ancient artifacts are found, scientists determine their age using a technique called radioactive dating. Carbon-14 is a radioactive isotope
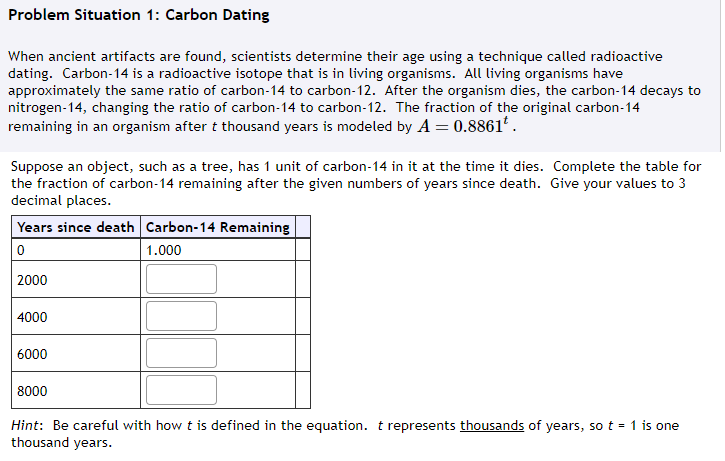
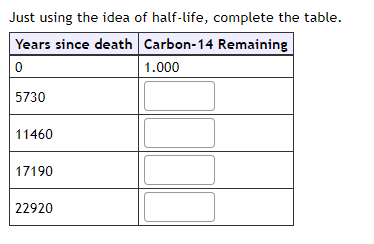
Problem Situation 1: Carbon Dating When ancient artifacts are found, scientists determine their age using a technique called radioactive dating. Carbon-14 is a radioactive isotope that is in living organisms. All living organisms have approximately the same ratio of carbon-14 to carbon-12. lifter the organism dies, the carbon-14 decays to nitrogen-14, changing the ratio of carbon-14 to carbon-12. The fraction of the original carbon-14 remaining in an organism after t thousand years is modeled bv A : H.8351t. Suppose an object, such as a tree, has 1 unit of carbon-14 in it at the time it dies. Complete the table for the fraction of carbon-14 remaining after the given numbers of vears since death. Give your values to 3 decimal places. Carbon-14 Remaining I Hint: Be careful with hov.r t is defined in the equation. t represents thousands of years, so t = 1 is one thousand years. Just using the idea of half-life, complete the table. Years since death Carbon-14 Remaining C 1.000 5730 11460 17190 22920
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


