Question: Problem Solving 2. Calculate the entropy change when graphite burns in sufficient supply of oxygen as shown in the following equation: Cgraphite+O2CO2(g)SSofCgraphitie(s)=5.7molKJSSofO2(g)=205molKJSSofCO2=213.6molKJ Question 32 Calculate
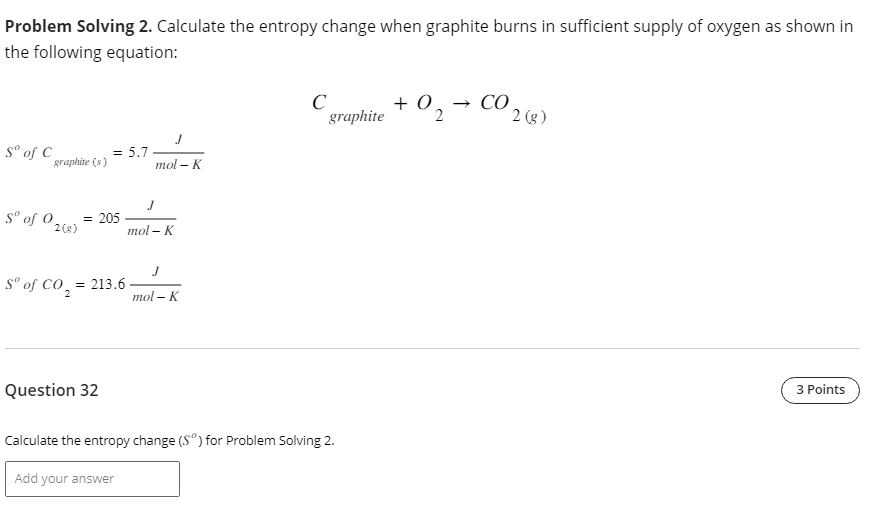
Problem Solving 2. Calculate the entropy change when graphite burns in sufficient supply of oxygen as shown in the following equation: Cgraphite+O2CO2(g)SSofCgraphitie(s)=5.7molKJSSofO2(g)=205molKJSSofCO2=213.6molKJ Question 32 Calculate the entropy change (S) for Problem Solving 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


