Question: Problem Three: (Based on Chapra, Problems 5.8) The saturation concentration of dissolved oxygen in freshwater can be calculated with the equation: In osf--139.344111.575701 x 105
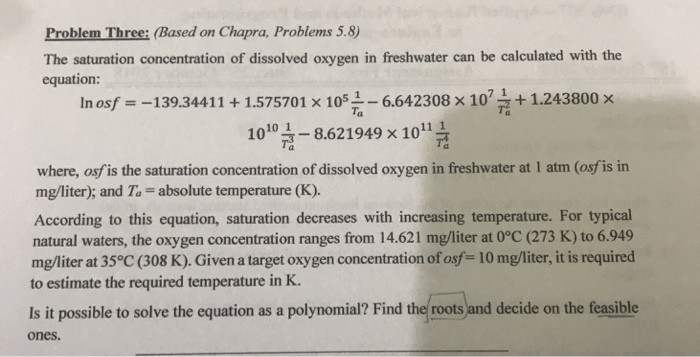
Problem Three: (Based on Chapra, Problems 5.8) The saturation concentration of dissolved oxygen in freshwater can be calculated with the equation: In osf--139.344111.575701 x 105 1-6.642308 x 101.243800 x 10101-86219498 1011h where, osfis the saturation concentration of dissolved oxygen in freshwater at 1 atm (osf is in mg/liter); and Ta -absolute temperature (K). According to this equation, saturation decreases with increasing temperature. For typical natural waters, the oxygen concentration ranges from 14.621 mg/liter at 0C (273 K) to 6.949 mg/liter at 35 c (308 K). Given a target oxygen concentration of osf 10 mg/liter, it is required to estimate the required temperature in K. Is it possible to solve the equation as a polynomial? Find the roots and decide on the feasible ones
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


