Question: Problem Two. (20 points) In this problem, we will model the CO molecule as a 3D rigid rotor. a. Given that a photon with frequency
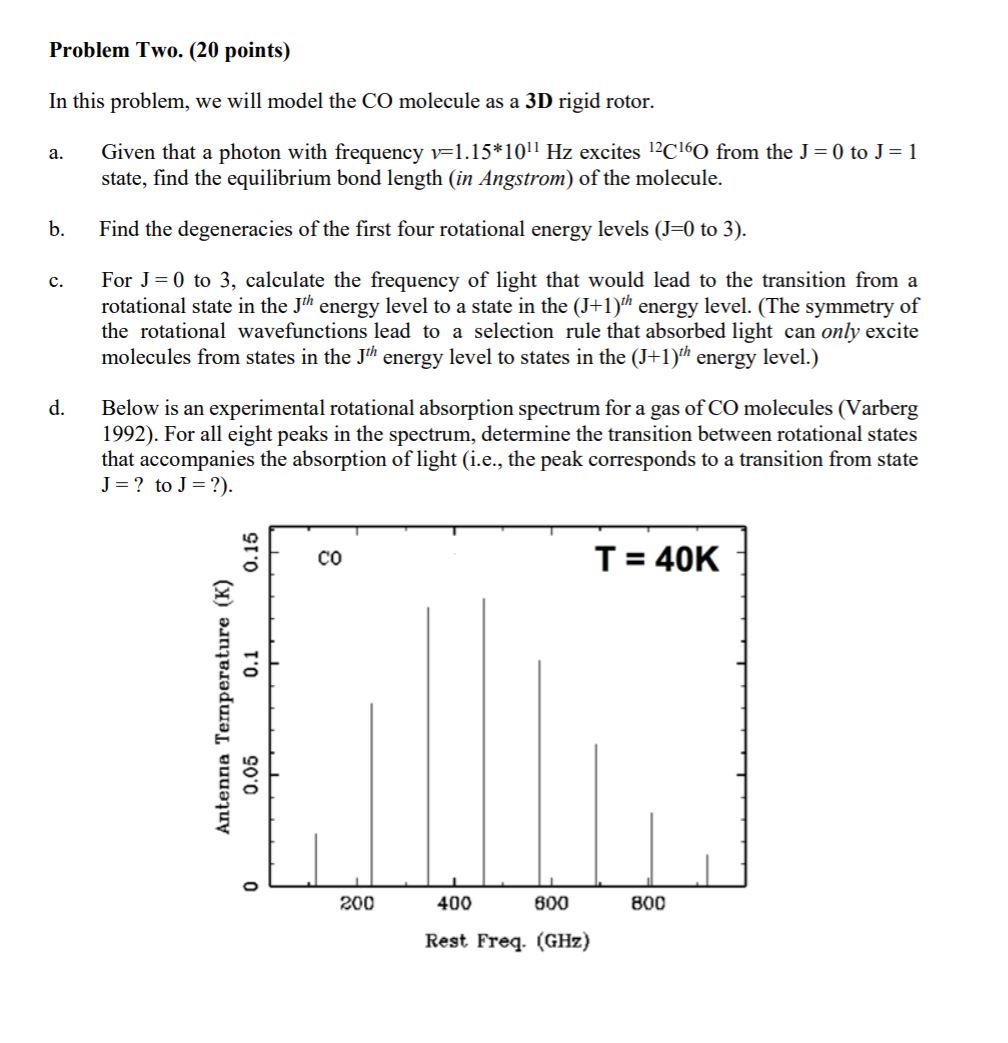
Problem Two. (20 points) In this problem, we will model the CO molecule as a 3D rigid rotor. a. Given that a photon with frequency v=1.15*1011 Hz excites 12C160 from the J=0 to J= 1 state, find the equilibrium bond length (in Angstrom) of the molecule. b. Find the degeneracies of the first four rotational energy levels (J=0 to 3). c. For J = 0 to 3, calculate the frequency of light that would lead to the transition from a rotational state in the Jth energy level to a state in the (5+1)th energy level. (The symmetry of the rotational wavefunctions lead to a selection rule that absorbed light can only excite molecules from states in the Jth energy level to states in the (5+1)th energy level.) d. Below is an experimental rotational absorption spectrum for a gas of CO molecules (Varberg 1992). For all eight peaks in the spectrum, determine the transition between rotational states that accompanies the absorption of light (i.e., the peak corresponds to a transition from state J= ? to J = ?). T = 40K 0.15 0.1 Antenna Temperature (K) 0.05 0 200 400 800 800 Rest Freq. (GHz) Problem Two. (20 points) In this problem, we will model the CO molecule as a 3D rigid rotor. a. Given that a photon with frequency v=1.15*1011 Hz excites 12C160 from the J=0 to J= 1 state, find the equilibrium bond length (in Angstrom) of the molecule. b. Find the degeneracies of the first four rotational energy levels (J=0 to 3). c. For J = 0 to 3, calculate the frequency of light that would lead to the transition from a rotational state in the Jth energy level to a state in the (5+1)th energy level. (The symmetry of the rotational wavefunctions lead to a selection rule that absorbed light can only excite molecules from states in the Jth energy level to states in the (5+1)th energy level.) d. Below is an experimental rotational absorption spectrum for a gas of CO molecules (Varberg 1992). For all eight peaks in the spectrum, determine the transition between rotational states that accompanies the absorption of light (i.e., the peak corresponds to a transition from state J= ? to J = ?). T = 40K 0.15 0.1 Antenna Temperature (K) 0.05 0 200 400 800 800 Rest Freq. (GHz)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


