Question: Problem Two. (35 points) Several processes performed on one mole of a monatomic, ideal gas are illustrated in the diagram below: D Ps.V. P.V. E
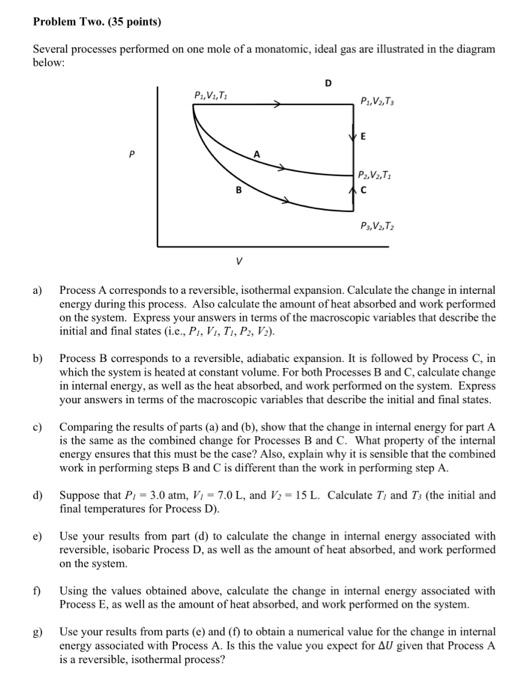
Problem Two. (35 points) Several processes performed on one mole of a monatomic, ideal gas are illustrated in the diagram below: D Ps.V. P.V. E P.V.: C P.V.T2 V a) Process A corresponds to a reversible, isothermal expansion. Calculate the change in internal energy during this process. Also calculate the amount of heat absorbed and work performed on the system. Express your answers in terms of the macroscopic variables that describe the initial and final states (i.c, P. V., T., P., V:). b) Process B corresponds to a reversible, adiabatic expansion. It is followed by Process C. in which the system is heated at constant volume. For both Processes B and C, calculate change in internal energy, as well as the heat absorbed, and work performed on the system. Express your answers in terms of the macroscopic variables that describe the initial and final states. c) Comparing the results of parts (a) and (b), show that the change in internal energy for part A is the same as the combined change for Processes B and C. What property of the internal energy ensures that this must be the case? Also, explain why it is sensible that the combined work in performing steps B and C is different than the work in performing step A. d) Suppose that P, = 3.0 atm, V, - 7.0 L, and V2 - 15 L. Calculate Tand T(the initial and final temperatures for Process D). e) Use your results from part (d) to calculate the change in intemal energy associated with reversible, isobaric Process D, as well as the amount of heat absorbed, and work performed on the system 1) Using the values obtained above, calculate the change in internal energy associated with Process E, as well as the amount of heat absorbed, and work performed on the system. 8) Use your results from parts (e) and () to obtain a numerical value for the change in internal energy associated with Process A. Is this the value you expect for AU given that Process A is a reversible, isothermal process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


