Question: Problems 1. Why should I-trispropan-2-yl)silylpyrrole react with N-bro- mosuccinimide (a source of Br) to give 3-bromo-1-ftris propan-2. y silylpyrrole, rather than the 2-isomer? 2. Suggest
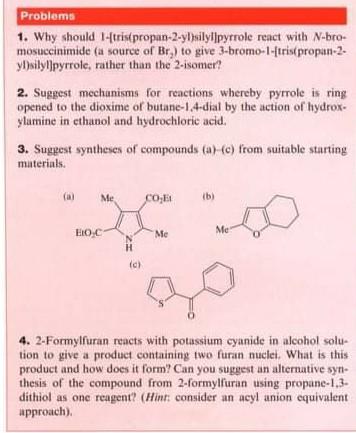
Problems 1. Why should I-trispropan-2-yl)silylpyrrole react with N-bro- mosuccinimide (a source of Br) to give 3-bromo-1-ftris propan-2. y silylpyrrole, rather than the 2-isomer? 2. Suggest mechanisms for reactions whereby pyrrole is ring opened to the dioxime of butane-1,4-dial by the action of hydrox. ylumine in ethanol and hydrochloric acid. 3. Suggest syntheses of compounds (a)-(c) from suitable starting materials. Me CO ib) Eroc Me Me H c) 4. 2-Formylfuran reacts with potassium cyanide in alcohol solu- tion to give a product containing two furan nuclei. What is this product and how does it form? Can you suggest an alternative synl thesis of the compound from 2-formylluran using propane-1,3- dithiol as one reagent? (Hint: consider an acyl anion equivalent approach)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


