Question: PROCEDURE 1. Access 8.1 - Introduction under the escience Tab and read all sections. 2. Complete 8.2 - Experiment 1: Ideal Gas Law - Finding
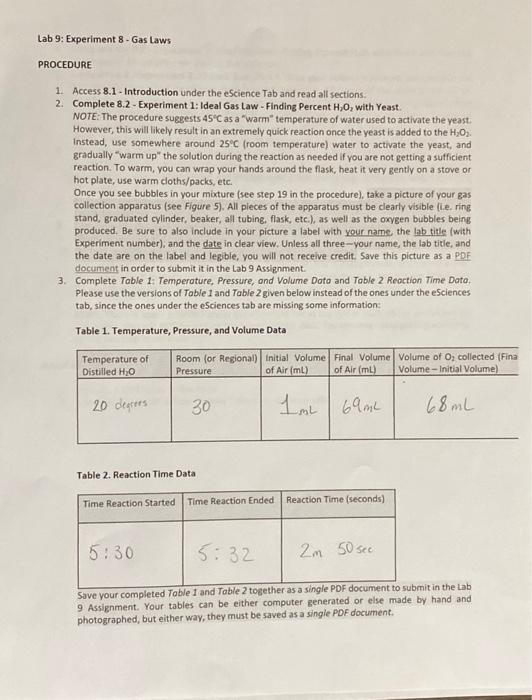
PROCEDURE 1. Access 8.1 - Introduction under the escience Tab and read all sections. 2. Complete 8.2 - Experiment 1: Ideal Gas Law - Finding Percent H2O2 with Yeast. NOTE: The procedure suggests 45C as a "Warm" temperature of water used to activate the yeast: However, this will likely result in an extremely quick reaction once the yeast is added to the H2O2. Instead, use somewhere around 25C (room temperature) water to activate the yeast, and gradually "warm up" the solution during the reaction as needed if you are not getting a sutficient reaction. To warm, you can wrap your hands around the flask, heat it very gently on a stove or hot plate, use warm cloths/packs, etc. Once you see bubbles in your maxture (see step 19 in the procedure), take a picture of your gas collection apparatus (see Figure 5). All pieces of the apparatus must be clearly visible (i.e. ring stand, graduated cylinder, beaker, all tubing, flask, etc.), as well as the oxygen bubbles being produced. Be sure to also include in your picture a label with your name, the lab title (with Experiment number), and the date in clear view. Unless all three-your name, the lab title, and the date are on the label and legible, you will not receive credit. Save this picture as a PDF documeat in order to submit it in the Lab 9 Assignment. 3. Complete Table 1: Temperature, Pressure, and Volume Doto and Table 2 Reoction Time Dato. Please use the versions of Table 1 and Table 2 given belowinstead of the ones under the eSciences tab, since the ones under the eSciences tab are missing some information: Table 1. Temperature, Pressure, and Volume Data Table 2. Reaction Time Data Save your completed Table 1 and Table 2 together as a singre pur oocumentw wubmit in the Lab 9 Assignment. Your tables can be either computer generated or else made by hand and photographed, but either way, they must be saved as a single PDF document. PROCEDURE 1. Access 8.1 - Introduction under the escience Tab and read all sections. 2. Complete 8.2 - Experiment 1: Ideal Gas Law - Finding Percent H2O2 with Yeast. NOTE: The procedure suggests 45C as a "Warm" temperature of water used to activate the yeast: However, this will likely result in an extremely quick reaction once the yeast is added to the H2O2. Instead, use somewhere around 25C (room temperature) water to activate the yeast, and gradually "warm up" the solution during the reaction as needed if you are not getting a sutficient reaction. To warm, you can wrap your hands around the flask, heat it very gently on a stove or hot plate, use warm cloths/packs, etc. Once you see bubbles in your maxture (see step 19 in the procedure), take a picture of your gas collection apparatus (see Figure 5). All pieces of the apparatus must be clearly visible (i.e. ring stand, graduated cylinder, beaker, all tubing, flask, etc.), as well as the oxygen bubbles being produced. Be sure to also include in your picture a label with your name, the lab title (with Experiment number), and the date in clear view. Unless all three-your name, the lab title, and the date are on the label and legible, you will not receive credit. Save this picture as a PDF documeat in order to submit it in the Lab 9 Assignment. 3. Complete Table 1: Temperature, Pressure, and Volume Doto and Table 2 Reoction Time Dato. Please use the versions of Table 1 and Table 2 given belowinstead of the ones under the eSciences tab, since the ones under the eSciences tab are missing some information: Table 1. Temperature, Pressure, and Volume Data Table 2. Reaction Time Data Save your completed Table 1 and Table 2 together as a singre pur oocumentw wubmit in the Lab 9 Assignment. Your tables can be either computer generated or else made by hand and photographed, but either way, they must be saved as a single PDF document
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


