Question: PROCEDURE: Part 1: Preparing the solutions Preparing the acid and base solutions 1. Using a graduated cylinder, measure 20mL of water and add it to

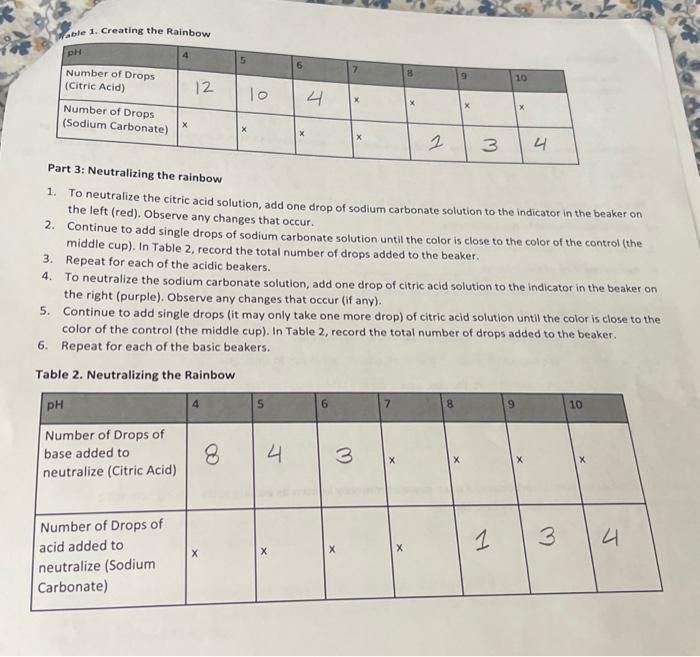

PROCEDURE: Part 1: Preparing the solutions Preparing the acid and base solutions 1. Using a graduated cylinder, measure 20mL of water and add it to a beaker labeled "Citric Acid Solution." When using a graduated cylinder, make sure to stoop down and measure with your eyes at the level of the water line. The bottom of the meniscus (see picture) should be at the 20mL line to accurately measure. 2. Using a graduated cylinder, measure 20ml of water and add it to a beaker labeled "Sodium Carbonate Solution." 3. Add ahe scoop of citric acid to a beaker labeled "citric Acid Solution (ACID)." Gently swirl the mixture until the citric acid dissolves. This is the citric acid solution. 4. Add tho scoops of sodium carbonate to a beaker labeled "Sodium Carbonate Solution (BASE)." Gently swirl the mixture until the sodium carbonate dissolves. This is the sodium carbonate solution. Diluting a universal indicator 1. Use a graduated cylinder to add 20mL of water each to seven beakers. 2. Add 10 drops of universal indicator to each beaker and gently swirl to mix thoroughly. 3. Line the beakers up horizontally in front of you. Part 2: Creating a Rainbow 1. Add one or two drops of citric acid solution to the beaker on the far left. Gently swirl the liquid in the beaker and observe. Continue adding drops (making sure to keep count of how many drops are added) and swirling until a pH4 solution is made. What color is a pH4 solution? 2. In Table 1, record the number of drops of citric acid used to make a pH4 solution. 3. Add one or two drops of sodium carbonate solution to the beaker on the far right. Gently swirl the liquid in the beaker and observe. Continue adding drops (making sure to keep count of how many drops are added) and swirling until a pH10 solution is made. What color is a pH10 solution? 4. In Table 1, record the number of drops of sodium carbonate used to make a pH 10 solution. 5. Using the remaining beakers, make solutions of pH5,6,8 and 9 using the method above. In Table 1 , record the number of drops used to create each of these four new solutions. Fart s: Neutralizing the rainbow 1. To neutralize the citric acid solution, add one drop of sodium carbonate solution to the indicator in the beaker on the left (red). Observe any changes that occur. 2. Continue to add single drops of sodium carbonate solution until the color is close to the color of the control (the middle cup). In Table 2, record the total number of drops added to the beaker. 3. Repeat for each of the acidic beakers. 4. To neutralize the sodium carbonate solution, add one drop of citric acid solution to the indicator in the beaker on the right (purple). Observe any changes that occur (if any). 5. Continue to add single drops (it may only take one more drop) of citric acid solution until the color is close to the color of the control (the middle cup). In Table 2 , record the total number of drops added to the beaker. 6. Repeat for each of the basic beakers. Table 2. Neutralizing the Rainbow How many drops of acid did you have to add to neutralize the pH10 solution? Was it more or less than the amount of drops you had to had to neutralize the pH 8 solution? Using what you know about pH, explain why this is the case. Mops More How many drops of base did you have to add to neutralize the pH 4 solution? Was it more or less than the amount of drops you had to had to neutralize the pH 6 solution? Using what you know about pH, explain why this is the case
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


