Question: Procedure This is a two-day lab. You will colloct all of your data during the firct day, and save any catculations and plotting for the
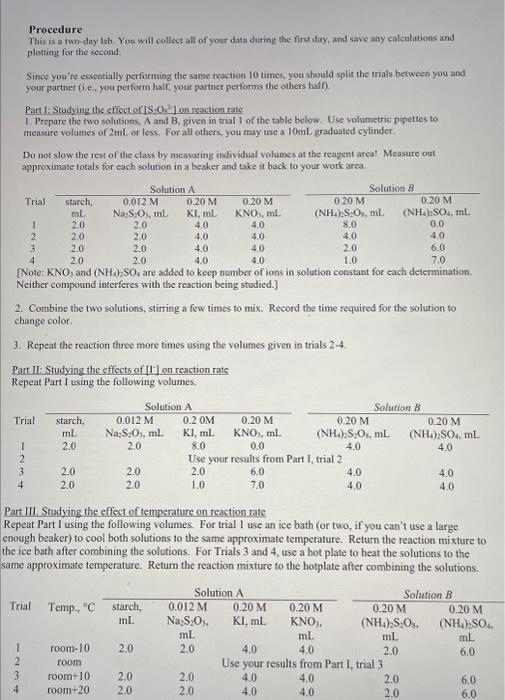

Procedure This is a two-day lab. You will colloct all of your data during the firct day, and save any catculations and plotting for the second. Since you're exsentially performing the same reaction 10 -imes, you should split the trials between you and your partner (i.e, you periorm half, your partner perfamms the others half). Part 1: Studying the effect of S3O8* - on reaction rate 1. Prepare the two solutions, A and B, given in trial I of the table below. Use volumetric pipeties to measure volumes of 2mL or less. For all others, you may use a 10mL graduated cylinder. Do not slow the rest of the class by meavaring individual volumes at the reagent area! Measure out approximate totals for each solution in a beaker and take it back to your work area. [Note: KNO3 and (NH4)2SO4 are added to keep number of ions in solution constant for each determination. Neither compound interferes with the reaction being studied.] 2. Combine the two solutions, stirring a few timas to mix. Record the time required for the solution to change color. 3. Repeat the reaction three more times using the volumes given in trials 24. Part 1I: Studying the effects of [1-] on reaction rate Repeat Part I using the following volumes. Part III. Studying the effect of temperature on reaction mate Repeat Part I using the following volumes. For trial I use an ice bath (or two, if you can't use a large enough beaker) to cool both solutions to the same approximate temperature. Return the reaction mixture to the ice bath after combining the solutions. For Trials 3 and 4 , use a bot plate to heat the solutions to the same approximate temperature. Retum the reaction mixture to the hotplate after combining the solutions. Part II: Studying the effects of [I] on reaction rate Trial 4) Moles of S2O72 5) Moles of I3 produced during time observed 6) [13]during time observed, ( (M) 7) Initial Rate, based on average time (M/s) 8) In Part II, find three sets of trials where the concentration of iodide is doubled. List each set below. a) Trials \& \& b) Trials \& c) Trials & 9) For each set of trials, determine the effect doubling concentration had on the reaction rate, rounding to the nearest integer. a) b) c) Show your work for 9a 10) For each set of trials, determine the reaction order with respect to iodide a) b) c) 11) Average order (rounded to nearest integer) 12) Based on your data from Parts I and II, what is the rate law for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


