Question: Process Synthesis QUESTION 2 Acrylic acid (AA) is the polymer for bulletproof glass. The production of AA using a zeolite-based catalyst from propylene partial oxidation
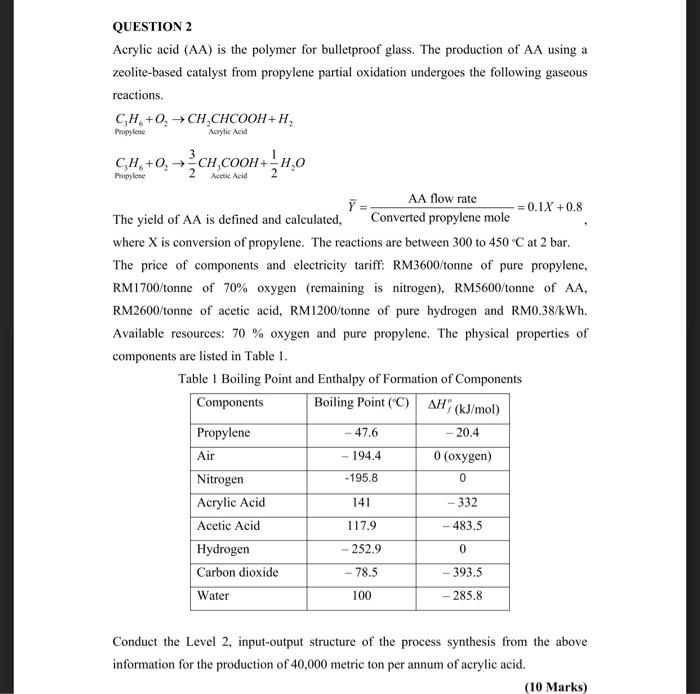
QUESTION 2 Acrylic acid (AA) is the polymer for bulletproof glass. The production of AA using a zeolite-based catalyst from propylene partial oxidation undergoes the following gaseous reactions. CH+0,-CH,CHCOOH + H, Propylene Acrylic Acid CH,+0,ch cooH+4:0 2 Y AA flow rate =0.1X +0.8 The yield of AA is defined and calculated, Converted propylene mole where X is conversion of propylene. The reactions are between 300 to 450C at 2 bar. The price of components and electricity tariff: RM3600/tonne of pure propylene, RM 1 700/tonne of 70% oxygen (remaining is nitrogen), RM5600 tonne of AA, RM2600/tonne of acetic acid, RM1200/tonne of pure hydrogen and RM0.38/kWh. Available resources: 70 % oxygen and pure propylene. The physical properties of components are listed in Table 1. Table 1 Boiling Point and Enthalpy of Formation of Components Components Boiling Point (C) AH(kJ/mol) Propylene - 20.4 Air 194.4 0 (oxygen) Nitrogen Acrylic Acid 141 - 332 Acetic Acid 117.9 -483.5 Hydrogen 252.9 0 Carbon dioxide 78.5 393.5 Water 100 285.8 -47.6 -195.8 0 - Conduct the Level 2, input-output structure of the process synthesis from the above information for the production of 40,000 metric ton per annum of acrylic acid. (10 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


