Question: Process Variables (refer to the Process Variables Handout for concepts and formulas) 3. (15 points) Twenty-five lbm of a gas mixture consisting of 30.0 %
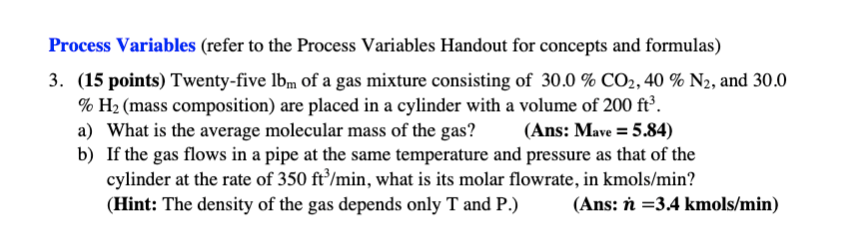
Process Variables (refer to the Process Variables Handout for concepts and formulas) 3. (15 points) Twenty-five lbm of a gas mixture consisting of 30.0 % CO2, 40 % N2, and 30.0 % H2 (mass composition) are placed in a cylinder with a volume of 200 ft. a) What is the average molecular mass of the gas? (Ans: Mave = 5.84) b) If the gas flows in a pipe at the same temperature and pressure as that of the cylinder at the rate of 350 ft/min, what is its molar flowrate, in kmols/min? (Hint: The density of the gas depends only T and P.) (Ans: n =3.4 kmols/min)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


