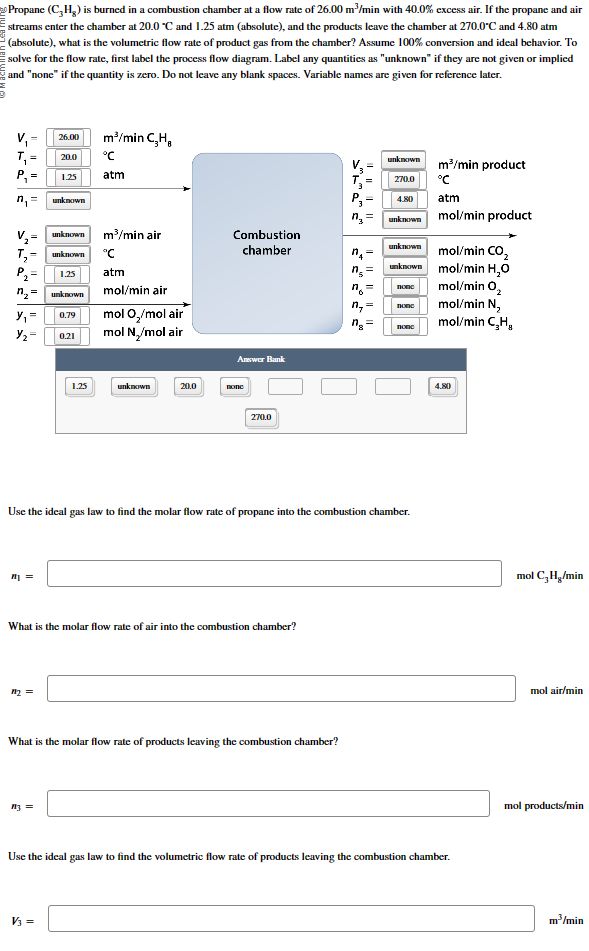
Question: = Propane ( C 3 H 8 ) is burned in a combustion chamber at a flow rate of 2 6 . 0 0 m
Propane is burned in a combustion chamber at a flow rate of with excess air. If the propane and air
streams enter the chamber at and atm absolute and the products leave the chamber at and atm
absolute what is the volumetric flow rate of product gas from the chamber? Assume conversion and ideal behavior. To
solve for the flow rate, first label the process flow diagram. Label any quantities as "unknown" if they are not given or implied
and "none" if the quantity is zero. Do not leave any blank spaces. Variable names are given for reference later.
Use the ideal gas law to find the molar flow rate of propane into the combustion chamber.
What is the molar flow rate of air into the combustion chamber?
What is the molar flow rate of products leaving the combustion chamber?
Use the ideal gas law to find the volumetric flow rate of products leaving the combustion chamber.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


