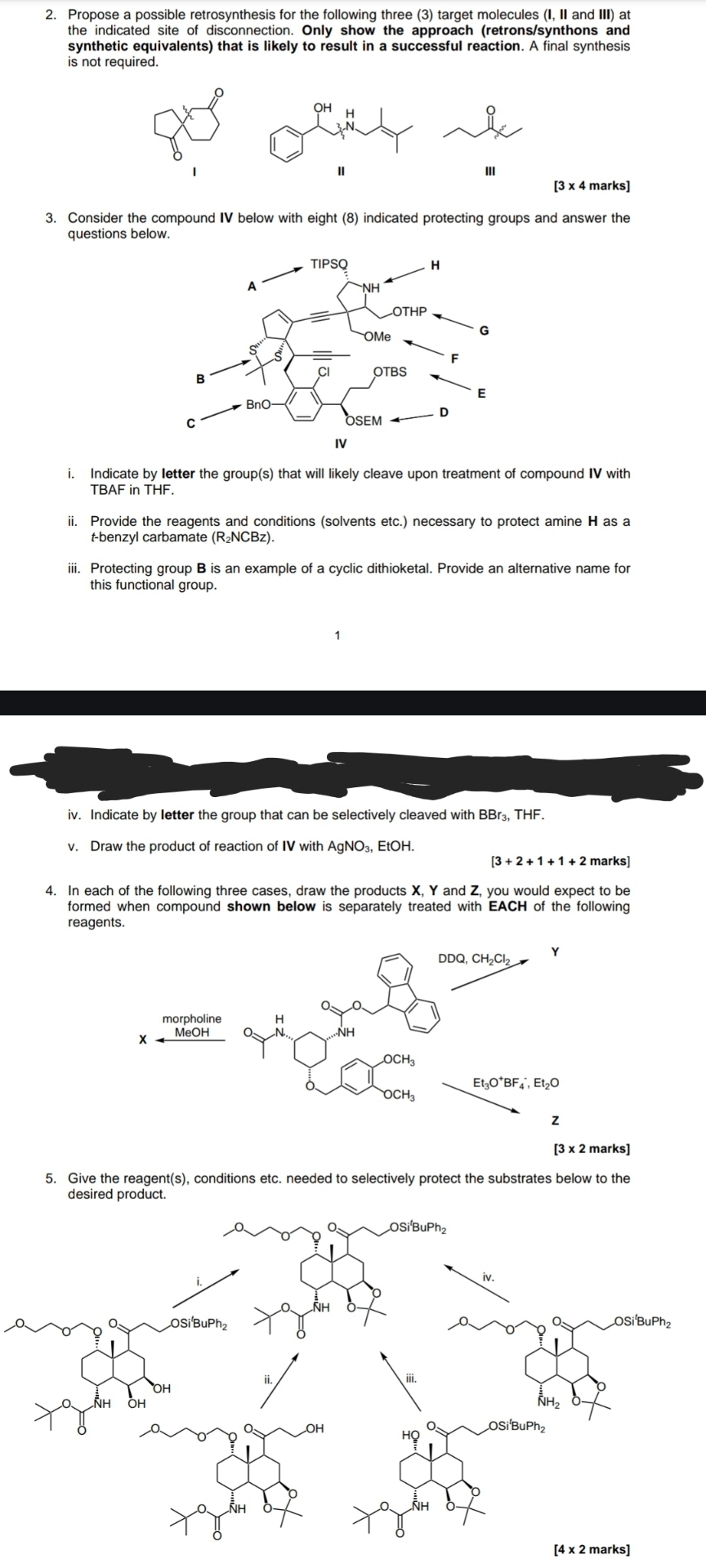
Question: Propose a possible retrosynthesis for the following three ( 3 ) target molecules ( I , II and III ) at the indicated site of
Propose a possible retrosynthesis for the following three target molecules I II and III at the indicated site of disconnection. Only show the approach retronssynthons and synthetic equivalents that is likely to result in a successful reaction. A final synthesis is not required.
marks
Consider the compound IV below with eight indicated protecting groups and answer the questions below.
i Indicate by letter the groups that will likely cleave upon treatment of compound IV with TBAF in THF
ii Provide the reagents and conditions solvents etc. necessary to protect amine as a benzyl carbamate
iii. Protecting group B is an example of a cyclic dithioketal. Provide an alternative name for this functional group.
iv Indicate by letter the group that can be selectively cleaved with
v Draw the product of reaction of IV with EtOH.
marks
In each of the following three cases, draw the products and you would expect to be formed when compound shown below is separately treated with EACH of the following reagents.
marks
Give the reagents conditions etc. needed to selectively protect the substrates below to the desired product.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


