Question: Prove that dS = ___ CV T ( T___ P ) V dP + ___ CP T ( T___ V ) P dV For an
Prove that dS = ___ CV T ( T___ P ) V dP + ___ CP T ( T___ V ) P dV For an ideal gas with constant heat capacities, use this result to derive Eq. (3.23c)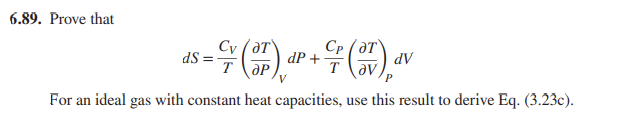
6.89. Prove that dS=TCV(PT)VdP+TCP(VT)PdV For an ideal gas with constant heat capacities, use this result to derive Eq. (3.23c)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


