Question: Prove the 2 questions step by step please In general for an arbitrary thermodynamic property of a pure substance, M=M(T,P); whence dM=(TM)PdT+(PM)TdP Prove that dS=TCV(PT)VdP+TCP(VT)PdV

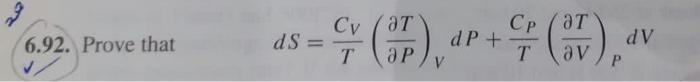
In general for an arbitrary thermodynamic property of a pure substance, M=M(T,P); whence dM=(TM)PdT+(PM)TdP Prove that dS=TCV(PT)VdP+TCP(VT)PdV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


