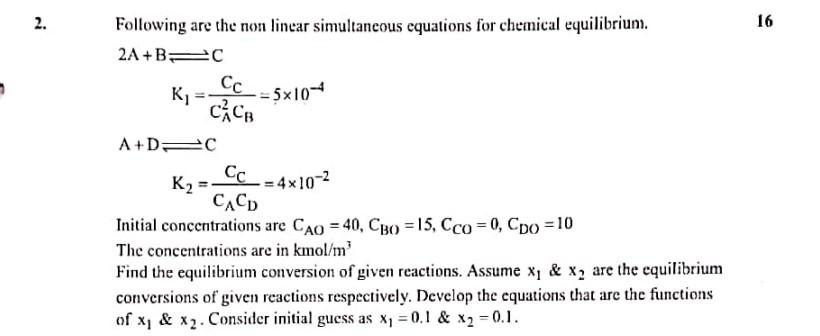
Question: provide answer in hand written notes Following are the non linear simultaneous equations for chemical equilibrium. 2A+BK1A+DK2C=CA2CBCC=5104C=CACDCC=4102 Initial concentrations are CAO=40,CBO=15,CCO=0,CDO=10 The concentrations are in
provide answer in hand written notes
Following are the non linear simultaneous equations for chemical equilibrium. 2A+BK1A+DK2C=CA2CBCC=5104C=CACDCC=4102 Initial concentrations are CAO=40,CBO=15,CCO=0,CDO=10 The concentrations are in kmol/m3 Find the equilibrium conversion of given reactions. Assume x1&x2 are the equilibrium conversions of given reactions respectively. Develop the equations that are the functions of x1&x2. Consider initial guess as x1=0.1&x2=0.1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


