Question: Provided data for Exp 11 and instructions for data analysis and lab report 1. Provided data for Exp 11 Table 1 Data from Measurement #1

![Measurement #1 with initial volume ], = 60 cc and 7 =](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6fdbdb5bc0_84566f6fdbd74e3b.jpg)
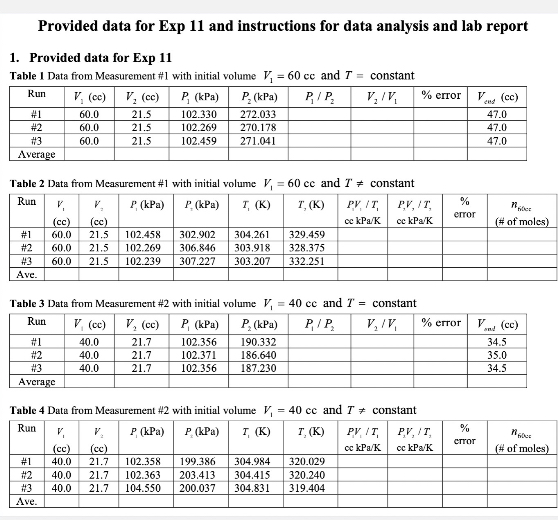
Provided data for Exp 11 and instructions for data analysis and lab report 1. Provided data for Exp 11 Table 1 Data from Measurement #1 with initial volume ], = 60 cc and 7 = constant Run V, (cc) V, (cc) P (kPa) P. (kPa) P /P, V, IV % erTOT #1 60.0 21.5 102.330 272.033 47.0 #2 60.0 21.5 102.269 270.178 47.0 60.0 21.5 102.459 271.041 47.0 Average Table 2 Data from Measurement #1 with initial volume 1 = 60 cc and ? = constant Run P (kPa) P (KP=) I (K) " (K) PV /T PV, IT error (cc) (cc) oc kPa/K cc kPa/K (# of moles) #1 60.0 21.5 102.458 302.902 304.261 329.459 #2 60.0 21.5 102.269 306.846 303.918 328.375 60.0 21.5 102.239 307.227 303.207 332.251 Ave. Table 3 Data from Measurement #2 with initial volume 1 = 40 cc and ' = constant Run V (cc) V, (CC) P (kPa) P. (kPa) PIP. V, /V % error V (cc) #1 10.0 21.7 102.356 190.332 34.5 #2 40.0 21.7 102.371 186.640 35.0 40.0 21.7 102.356 187.230 34.5 Average Table 4 Data from Measurement #2 with initial volume 1 = 40 cc and 7 * constant Run V. P (kPa) P (kPa) I (K) 7 (K) PV /I PV IT STTOT [cc) (cc) cc kPa/K oc kPa/K (# of moles) #1 40.0 21.7 102.358 199.386 304.984 320.029 #2 40.0 21.7 102.363 203.413 304.415 320.240 40.0 21.7 104.550 200.037 304.831 319.404 Ave.Questions 1. Complete Table 5. Table 5. Temperature units conversion Units name Value symbol Celsius .C Kelvin 2. Use the data in Tables 2 and 4 calculate the ratio: ngocc / 40ce . Is it equal to 60 cc / 40cc=1.5 ? Why? 3. Which of V, and 2 contributes more to the "% difference" in Table 1? Why? 4. Data in Tables 1 and 3 show that Vend # V,, why? 5. Based on the curves in Fig. 4, answer the following questions. (a) What happened to the temperature when the air was compressed? Why?(b) What is the equilibrium temperature of the gas when the plunger was compressed and before released? Why? (c) What happened to the temperature during the expansion (when you release the plunger) and why? Does it go below Ti and why? Does the pressure go below P, and why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


