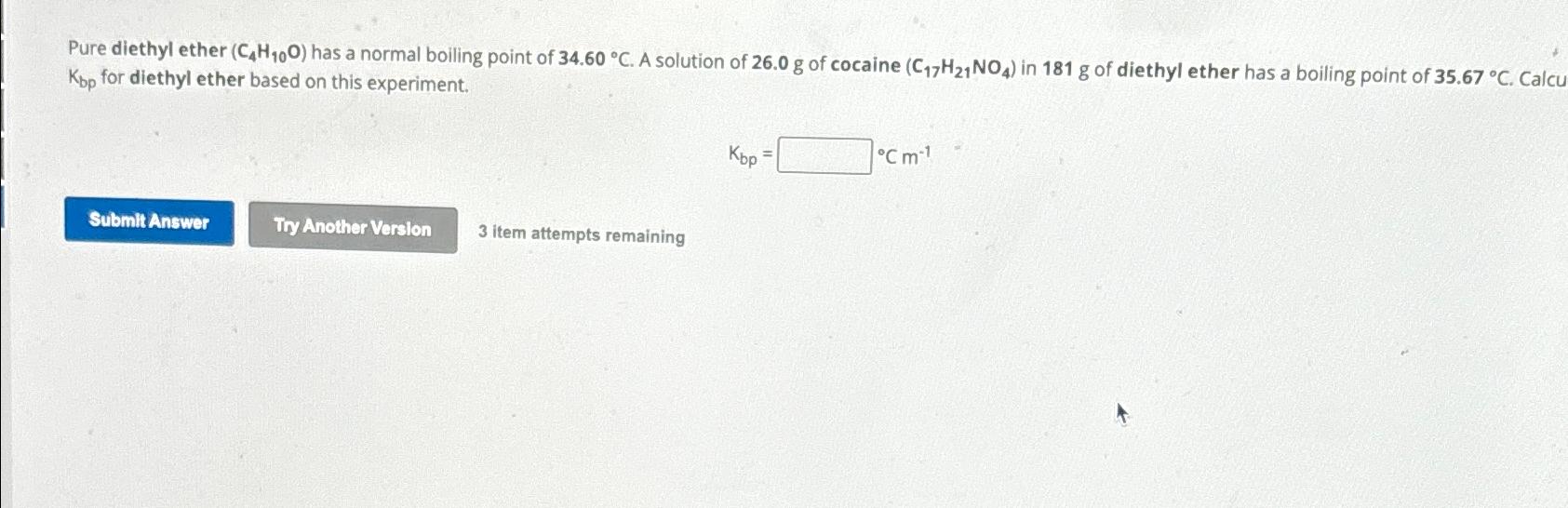
Question: Pure diethyl ether ( C 4 H 1 0 O ) has a normal boiling point of 3 4 . 6 0 C . A
Pure diethyl ether has a normal boiling point of A solution of of cocaine in of diethyl ether has a boiling point of Calcu for diethyl ether based on this experiment.
item attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


