Question: PV BP Z=- =1+ RT RT (3.36) 1) For the Nz(1)/CH4(2) mixture system and conditions in Example 10.7 find the parameter B in Eq. (3.36),


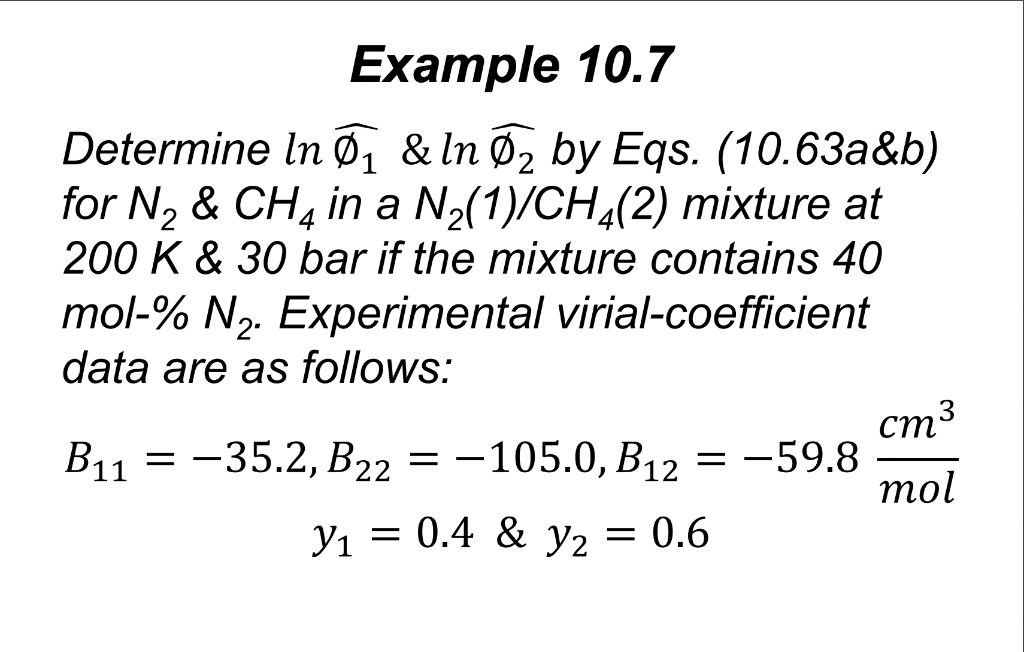
PV BP Z=- =1+ RT RT (3.36) 1) For the Nz(1)/CH4(2) mixture system and conditions in Example 10.7 find the parameter B in Eq. (3.36), compressibility factor Z and molar volume V for the mixture. Compare the molar volume to ideal gas molar volume and comment on both results. 2) Find the fugacities of components Ny & CH4 Example 10.7 Determine In , & ln 2 by Eqs. (10.63a&b) for N, & CH4 in a Nz(1)/CH4(2) mixture at 200 K & 30 bar if the mixture contains 40 mol-% N2. Experimental virial-coefficient data are as follows: cm3 = = Bu = -35.2, B22 = -105.0, B12 = -59.8 mol y = 0.4 & yz = 0.6 = =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


