Question: Python 3 In 1913 Bohr presented the first atomic model, where electrons orbit around a positively charged nucleus at the centre of the atom. In
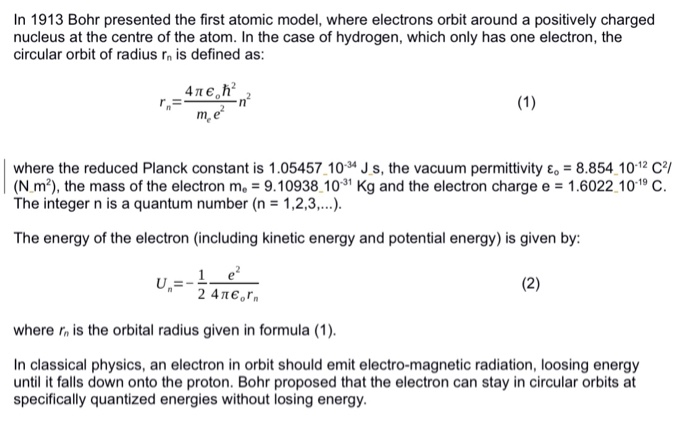

In 1913 Bohr presented the first atomic model, where electrons orbit around a positively charged nucleus at the centre of the atom. In the case of hydrogen, which only has one electron, the circular orbit of radius rn is defined as: m, e where the reduced Planck constant is 1.05457.104 J s, the vacuum permittivity &-8.854 1012 C (N m2), the mass of the electron me-9.10938 103 Kg and the electron charge e 1.6022.1019 C The integer n is a quantum number (n 1,2,3,..). The energy of the electron (including kinetic energy and potential energy) is given by 24Teon where rn is the orbital radius given in formula (1) In classical physics, an electron in orbit should emit electro-magnetic radiation, loosing energy until it falls down onto the proton. Bohr proposed that the electron can stay in circular orbits at specifically quantized energies without losing energy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


