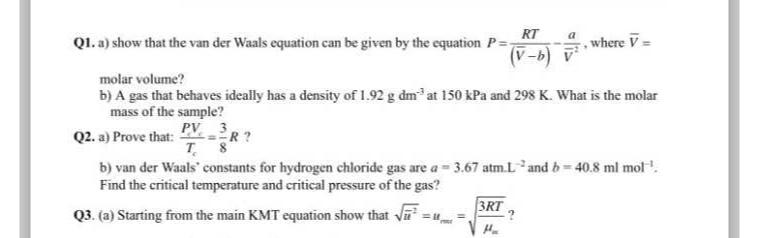
Question: Q 1 . a ) show that the van der Waals equation can be given by the equation P = R T ( ( ?
Q a show that the van der Waals equation can be given by the equation where molar volume?
b A gas that behaves ideally has a density of at kPa and What is the molar mass of the sample?
Q a Prove that: Find the critical temperature and critical pressure of the gas?
Qa Starting from the main KMT equation show that
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


