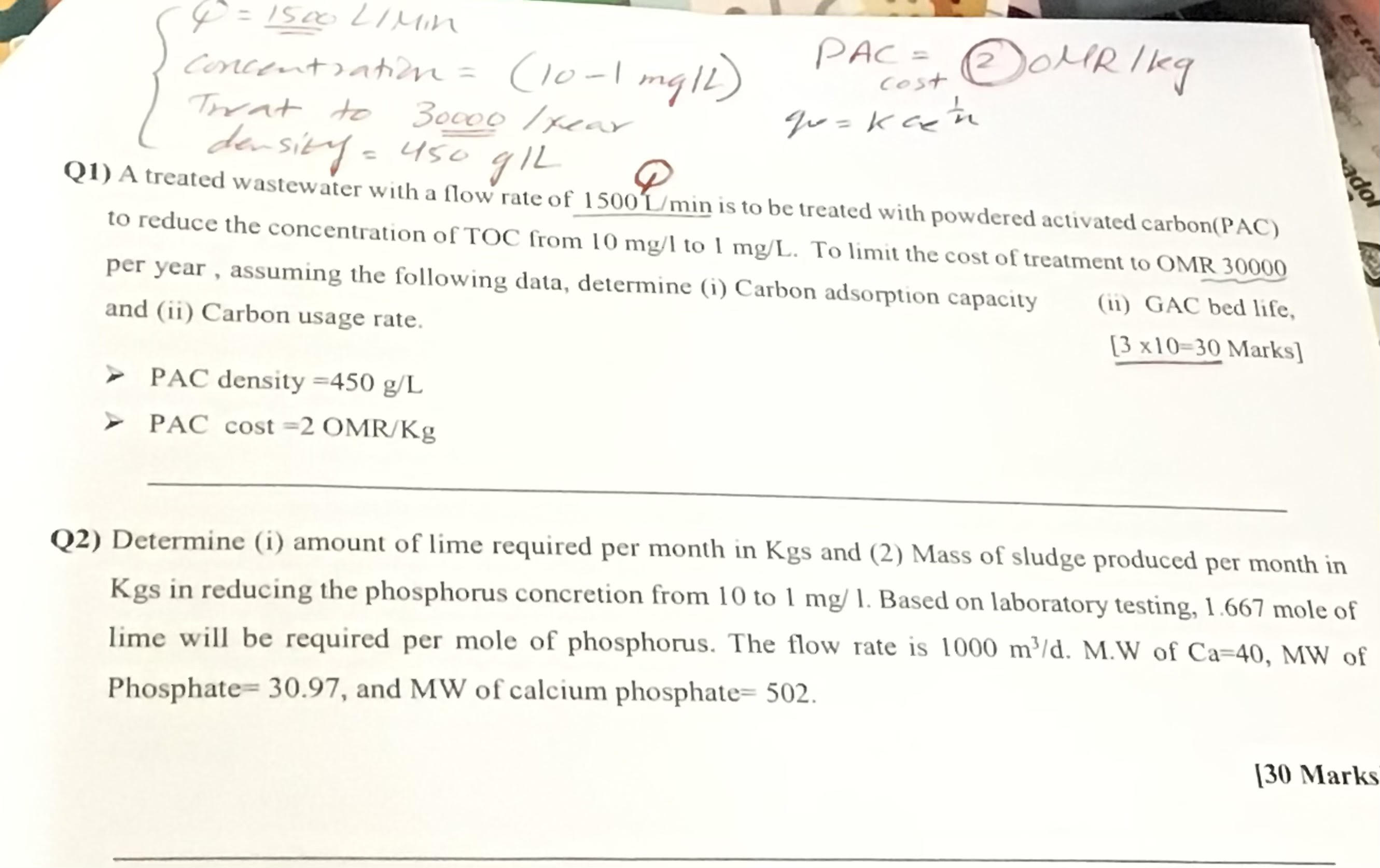
Question: Q 2 { [ 4 = 1 5 0 0 ) 2 1 Min concentiation = ( 1 0 - 1 m g L )
Q
Min
concentiation PAC
Q A treated wastewater with a flow rate of is to be treated with powdered activated carbonPAC
to reduce the concentration of TOC from to To limit the cost of treatment to OMR
per year, assuming the following data, determine i Carbon adsorption capacity
ii GAC bed life,
and ii Carbon usage rate.
Marks
PAC density
PAC cost
Q Determine i amount of lime required per month in Kgs and Mass of sludge produced per month in
in reducing the phosphorus concretion from to Based on laboratory testing, mole of
lime will be required per mole of phosphorus. The flow rate is MW of Ca MW of
Phosphate and MW of calcium phosphate
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


