Question: Q ( 2 ) Show that, when two different gases A and B of volume V A and V B and with N A and
Q Show that, when two different gases A and of volume and and with and molecules respectively are mixed together at constant temperature to form a volume there is an increase in the total entropy given by the mixing term What is your answer if
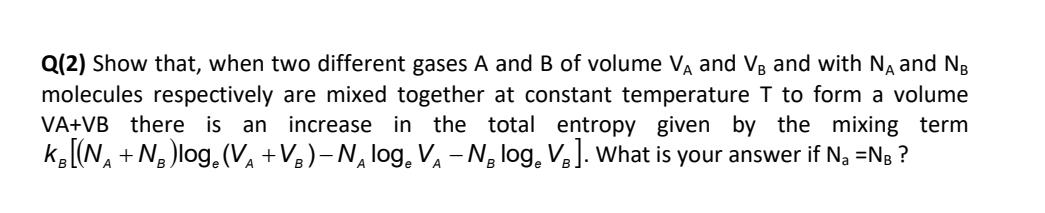
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


