Question: Q 3 ( 3 0 pts ) . Consider a 5 0 . 0 m L of 0 . 1 0 0 M sodium hydrogen
Q pts Consider a of sodium hydrogen oxalate NaHA solution.
For oxalic acid
a What is the of the solution?
b What will be the when of MNaOH is added to of MNaHA solution?
c What will be the when of is added to of MNaHA solution?
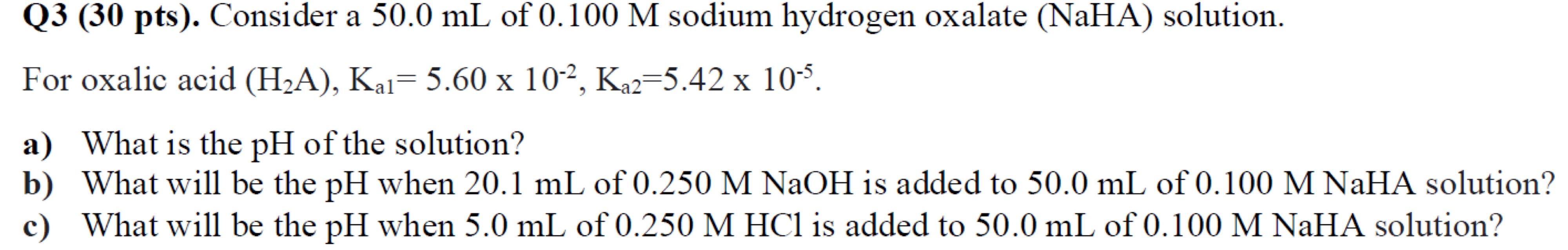
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


