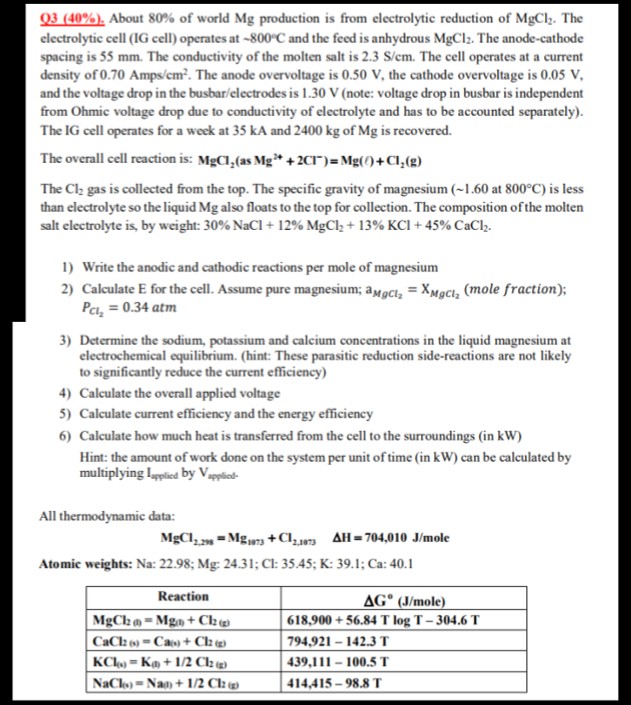
Question: Q 3 ( 4 0 % ) . About 8 0 % of world M g production is from electrolytic reduction of M g C
Q About of world production is from electrolytic reduction of The
electrolytic cell IG cell operates at and the feed is anhydrous The anodecathode
spacing is The conductivity of the molten salt is The cell operates at a current
density of Amp The anode overvoltage is the cathode overvoltage is
and the voltage drop in the busbarelectrodes is note: voltage drop in busbar is independent
from Ohmic voltage drop due to conductivity of electrolyte and has to be accounted separately
The IG cell operates for a week at and of is recovered.
The overall cell reaction is: as :
The gas is collected from the top. The specific gravity of magnesium at : is less
than electrolyte so the liquid also floats to the top for collection. The composition of the molten
salt electrolyte is by weight: NaCl
Write the anodic and cathodic reactions per mole of magnesium
Calculate for the cell. Assume pure magnesium; mole fraction;
atm
Determine the sodium, potassium and calcium concentrations in the liquid magnesium at
electrochemical equilibrium. hint: These parasitic reduction sidereactions are not likely
to significantly reduce the current efficiency
Calculate the overall applied voltage
Calculate current efficiency and the energy efficiency
Calculate how much heat is transferred from the cell to the surroundings in
Hint: the amount of work done on the system per unit of time in can be calculated by
multiplying by
All thermodynamic data:
ole
Atomic weights: :;:;:;:;:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


