Question: Q # 3. Consider the irreversible, liquid-phase (constant-volume), homogeneous rxn in a batch reactor: A+2B3C+D The kinetics of this reaction is given by rA=kCA2CB/CD where
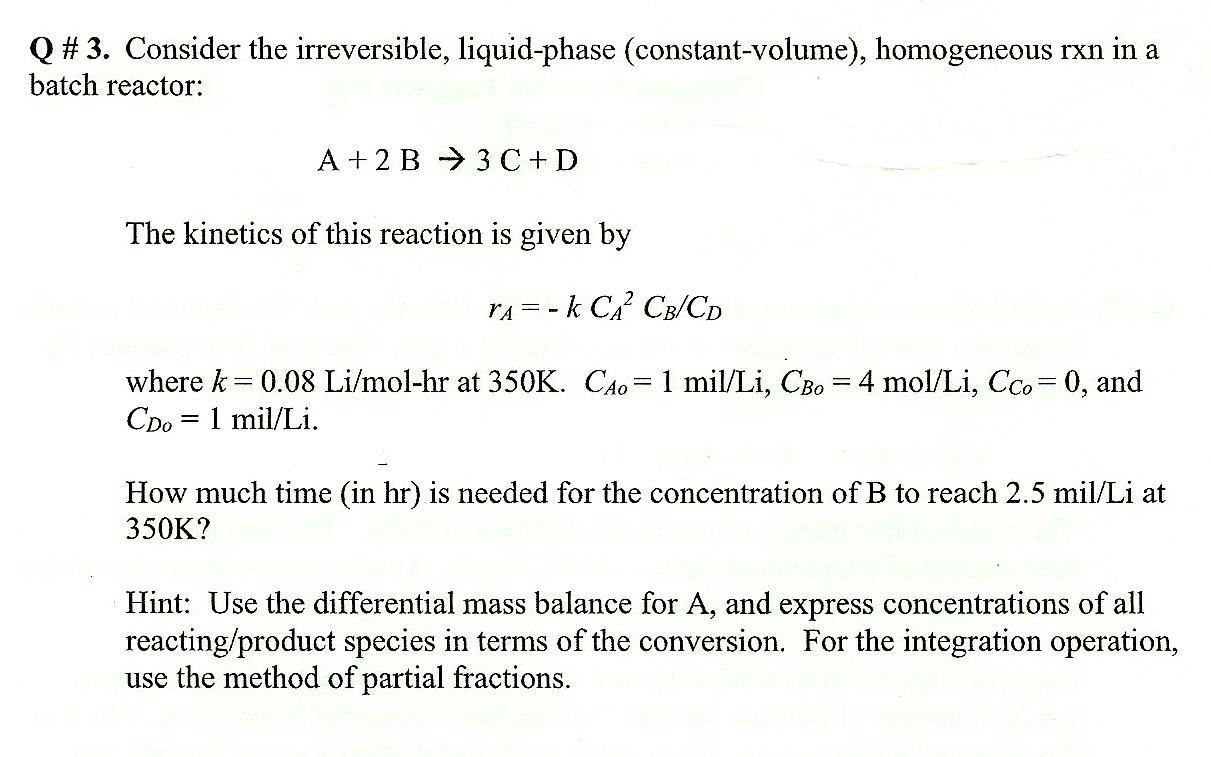
Q \# 3. Consider the irreversible, liquid-phase (constant-volume), homogeneous rxn in a batch reactor: A+2B3C+D The kinetics of this reaction is given by rA=kCA2CB/CD where k=0.08Li/molhr at 350K.CAo=1mil/Li,CBo=4mol/Li,CCo=0, and CDo=1mil/Li. How much time (in hr) is needed for the concentration of B to reach 2.5mil/Li at 350K ? Hint: Use the differential mass balance for A, and express concentrations of all reacting/product species in terms of the conversion. For the integration operation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


