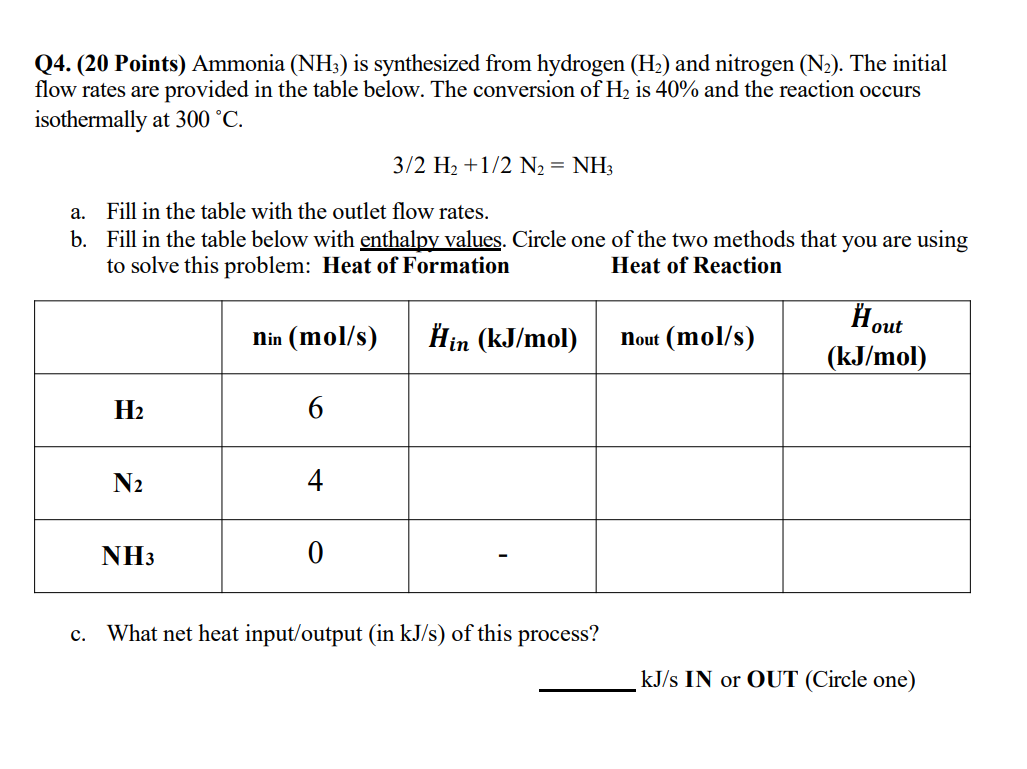
Question: Q 4 . ( 2 0 Points ) Ammonia ( N H 3 ) is synthesized from hydrogen ( H 2 ) and nitrogen (
Q Points Ammonia is synthesized from hydrogen and nitrogen The initial flow rates are provided in the table below. The conversion of is and the reaction occurs isothermally at
a Fill in the table with the outlet flow rates.
b Fill in the table below with enthalpy values. Circle one of the two methods that you are using to solve this problem: Heat of Formation
Heat of Reaction
tabletable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


