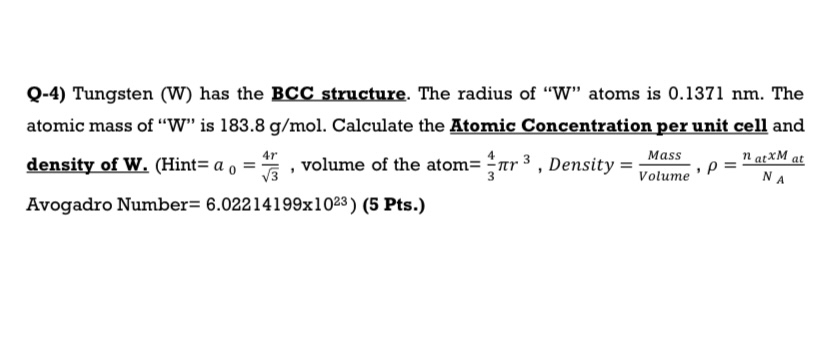
Question: Q - 4 ) Tungsten ( W ) has the BCC structure. The radius of W atoms is 0 . 1 3 7
Q Tungsten W has the BCC structure. The radius of W atoms is nm The atomic mass of W is Calculate the Atomic Concentration per unit cell and density of WHint volume of the atom Density Avogadro Number Pts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


