Question: Q1: In a particular installation, the purification system for the removal of sulfur compounds, designed to operate at a feed rate of up to 820
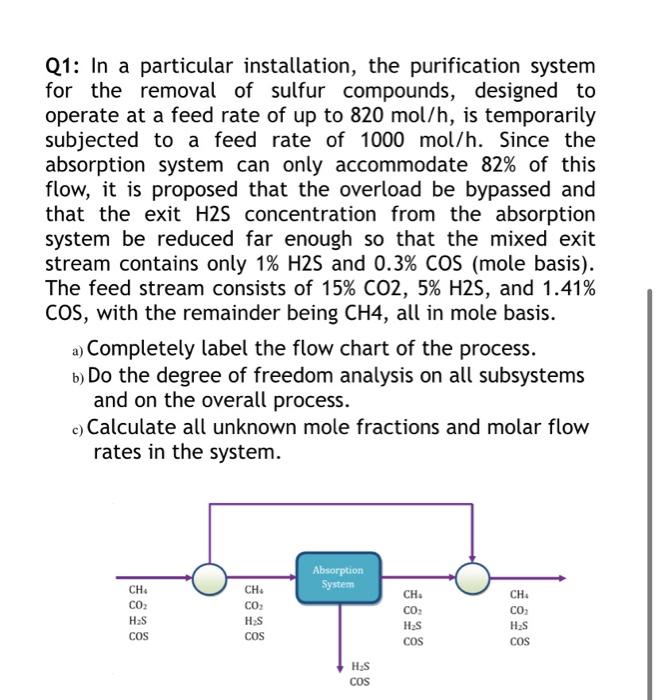
Q1: In a particular installation, the purification system for the removal of sulfur compounds, designed to operate at a feed rate of up to 820 mol/h, is temporarily subjected to a feed rate of 1000 mol/h. Since the absorption system can only accommodate 82% of this flow, it is proposed that the overload be bypassed and that the exit H2S concentration from the absorption system be reduced far enough so that the mixed exit stream contains only 1% H2S and 0.3% COS (mole basis). The feed stream consists of 15% CO2, 5% H2S, and 1.41% COS, with the remainder being CH4, all in mole basis. a) Completely label the flow chart of the process. b) Do the degree of freedom analysis on all subsystems and on the overall process. c) Calculate all unknown mole fractions and molar flow rates in the system. Absorption System CH. COM H2S COS CH. COM HS COS CH. CO HES cos CH CO HES COS H.S COS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


