Question: Q2: Second Order Reaction Carried Out Adiabatically in a CSTR. The acid-catalyzed irreversible liquid-phase reaction is: A - B The feed, which is equimolar in
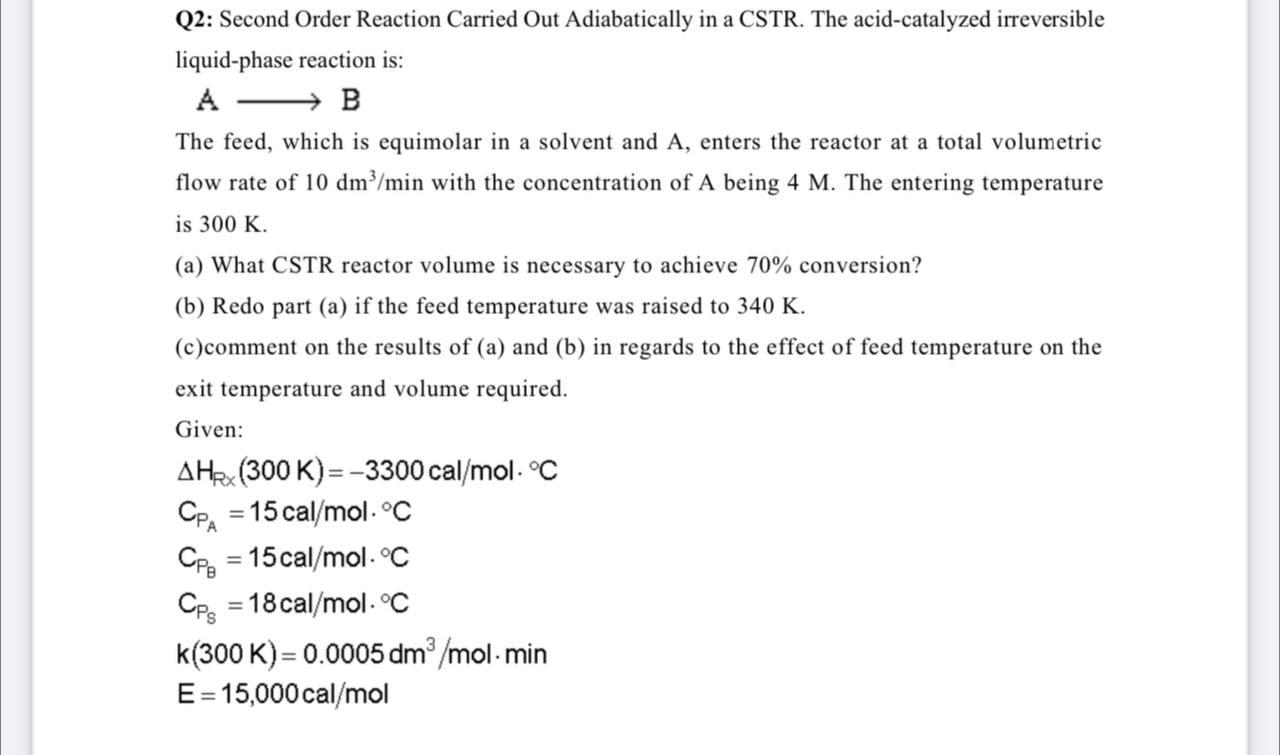
Q2: Second Order Reaction Carried Out Adiabatically in a CSTR. The acid-catalyzed irreversible liquid-phase reaction is: A - B The feed, which is equimolar in a solvent and A, enters the reactor at a total volumetric flow rate of 10 dm/min with the concentration of A being 4 M. The entering temperature is 300 K. (a) What CSTR reactor volume is necessary to achieve 70% conversion? (b) Redo part (a) if the feed temperature was raised to 340 K. (c)comment on the results of (a) and (b) in regards to the effect of feed temperature on the exit temperature and volume required. Given: AHRx (300 K)=-3300 cal/mol. C Cpa = 15 cal/mol.C Cpa = 15 cal/mol. C Cps = 18 cal/mol. C k(300 K)= 0.0005 dm3/mol. min E= 15,000 cal/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


